Infrared-Absorbing Gases and
the Earth's Surface Temperature:
A
Relatively Simple Baseline Evaluation of the Physics
Charles
R. Anderson, Ph.D., Physics
Introduction
This analysis of the Earth’s surface temperature will examine the case of an Earth in radiative equilibrium with space, assuming a constant solar insolation as the critical radiative source of energy. It will evaluate the role of the so-called greenhouse gases, which are really infrared absorbing and emitting gases, in our atmosphere in establishing the surface temperature of the Earth. The emphasis will be on examining these long-term baseline equilibrium effects. Clearly the sun has solar cycles, cooling cloud cover varies greatly, and the oceans with their huge heat content and slow response times to changes in solar insolation have their cycles also. These are terribly important effects, but they are not primary to the evaluation of the claim that increases in carbon dioxide in the atmosphere will lead to a catastrophic global warming. The examination of the basic physics undertaken here will provide a baseline understanding in terms of relatively simple physics of the role and effects of infrared absorbing and emitting gases generally within a dense atmosphere almost entirely composed of infrared-inactive gases. This paper will point out that the Earth’s surface is not in radiative equilibrium with space, though the Earth as a whole is. The fact that the atmosphere is dense, composed almost entirely of IR-inactive gases, and the role of water are the key facts in understanding the basic physics that determines the surface temperature of the Earth.
This analysis of the Earth’s surface temperature will examine the case of an Earth in radiative equilibrium with space, assuming a constant solar insolation as the critical radiative source of energy. It will evaluate the role of the so-called greenhouse gases, which are really infrared absorbing and emitting gases, in our atmosphere in establishing the surface temperature of the Earth. The emphasis will be on examining these long-term baseline equilibrium effects. Clearly the sun has solar cycles, cooling cloud cover varies greatly, and the oceans with their huge heat content and slow response times to changes in solar insolation have their cycles also. These are terribly important effects, but they are not primary to the evaluation of the claim that increases in carbon dioxide in the atmosphere will lead to a catastrophic global warming. The examination of the basic physics undertaken here will provide a baseline understanding in terms of relatively simple physics of the role and effects of infrared absorbing and emitting gases generally within a dense atmosphere almost entirely composed of infrared-inactive gases. This paper will point out that the Earth’s surface is not in radiative equilibrium with space, though the Earth as a whole is. The fact that the atmosphere is dense, composed almost entirely of IR-inactive gases, and the role of water are the key facts in understanding the basic physics that determines the surface temperature of the Earth.
Unfortunately, even at this
baseline level of understanding, the usual explanations of the basic physics as
rendered in the popular science media, government websites and publications, most
newspapers and magazines, TV, many global warming alarmist websites, most high
school science classes, most college courses, and even advocated by many
scientific professional societies are terribly wrong. The understanding here will make it clear
that it is unlikely that higher concentrations of carbon dioxide will have
catastrophic effects due to significant warming of the Earth’s surface. This baseline understanding will make it
clear that the advocates of such a hypothesis have failed to prove that very
dubious hypothesis with its critically important implications for our daily
lives, both in terms of our freedoms and our standard of living.
It is claimed by the
catastrophic man-made global warming advocates that infrared (IR) absorbing
water vapor, carbon dioxide, and methane gas, misleadingly called greenhouse
gases, are responsible for greatly warming the surface of the Earth. Measurements of radiation power from the
Earth, which include radiation from the Earth’s surface and the atmosphere with their respective temperatures and reflected solar radiation in the same frequency
ranges are commonly associated with a black body radiator that would produce
the same radiative power. From such a
calculation, as seen from space, the Earth has an effective “black body” radiation
temperature of about 255 Kelvin, abbreviated as 255K or -18ºC. The
actual radiation spectrum from Earth into space does not look like the
spectrum of a black body radiator at the temperature of 255K. The altitude in the U.S. Standard Atmosphere Table of 1976 with a temperature of 255K is 5100 meters. The Earth’s surface has an average
temperature commonly said to be about 287.65K or 14.5ºC. The difference in these temperatures of about
32.65K or 32.65º C is very commonly attributed to the so-called greenhouse gas effect. When this difference is assumed to be due to the greenhouse warming effect, it is a big effect.
This paper will show that the
Earth’s surface temperature would be much warmer than 255K in any case given
that the Earth’s surface is not actually a black body radiator and is not in radiative equilibrium with space. The substantial temperature gradient in the
lower atmosphere due to gravity will be calculated and discussed. It will note that the huge heat capacity of
the oceans, the land surface, the subsurface materials, and the atmosphere
itself is another warming effect over the daily cycle due to the effective
reduction of infrared (IR) radiative cooling of the Earth’s surface averaged
over the daily cycle. The combination of
the gravitational temperature gradient of the lower atmosphere due to the
combination of IR-absorbing and emitting gases and the density of the
atmosphere provided by IR-inactive gases provides a large warming effect upon
the Earth’s surface compared to the supposed 32.65K discrepancy with the Earth’s
radiative temperature as seen from space.
IR-absorbing gases play a significant role in determining the surface temperature of the Earth and in the distribution of heat within the atmosphere. But, this role is almost entirely due to water vapor in the lowest part of the atmosphere, the troposphere. This role of water vapor only exists because Earth is a water-covered planet. Water also plays a critical role in cooling and moderating the temperature of the surface of the Earth by evaporation and sublimation, by lightening the air to increase convection, by increasing the specific heat of the air, by forming clouds, and by condensing in clouds to form ice and water droplets with the release of great quantities of heat, which causes cloud expansion with further cooling of an enlarged shadowed surface area. Water vapor and carbon dioxide also have underrated roles in absorbing solar insolation in the atmosphere and preventing solar incoming IR radiation from warming the surface to what actually might be catastrophically high temperatures.
IR-absorbing gases play a significant role in determining the surface temperature of the Earth and in the distribution of heat within the atmosphere. But, this role is almost entirely due to water vapor in the lowest part of the atmosphere, the troposphere. This role of water vapor only exists because Earth is a water-covered planet. Water also plays a critical role in cooling and moderating the temperature of the surface of the Earth by evaporation and sublimation, by lightening the air to increase convection, by increasing the specific heat of the air, by forming clouds, and by condensing in clouds to form ice and water droplets with the release of great quantities of heat, which causes cloud expansion with further cooling of an enlarged shadowed surface area. Water vapor and carbon dioxide also have underrated roles in absorbing solar insolation in the atmosphere and preventing solar incoming IR radiation from warming the surface to what actually might be catastrophically high temperatures.
The natural effects of liquid
water and water vapor dwarf the effect of further additions by man to the rare IR-absorbing
gases of CO2, methane (CH4), and nitrous oxide (N2O). I
will provide reasons why these rare IR-absorbing gases are much less effective
in providing back-emitted IR radiation originally from the surface which the
surface can absorb than is water vapor.
I will also point out why water vapor is itself less effective in
warming the surface by re-emitted IR radiation it has absorbed from surface IR
emission than is usually thought to be the case by the catastrophic man-made
global warming advocates. The effect of
IR radiation from the atmosphere upon the surface temperature has been
generally greatly over-estimated while the size of the natural effects of the
previous paragraph has been greatly underestimated.
I will show that the essential physics can be summarized as:
Greenhouse Gas Hypotheses
I will show that the essential physics can be summarized as:
- Infra-red active (so-called greenhouse) gases absorb a substantial portion of the incoming solar radiation in the infrared portion of its spectrum with the result that additions to their concentrations have a cooling effect
- The Earth's surface is not a black body radiator, so it takes much less absorbed solar radiation to warm it to 287.65K or 14.5ºC than the alarmist greenhouse gas theories claim. In fact, the Earth's surface is only about half as efficient an infrared radiator as is a black body.
- The Stefan-Boltzmann law of radiation applies to a surface radiating into vacuum, not into an atmosphere able to provide competing cooling processes due to air conduction, air convection, and water evaporation. This Stefan-Boltzmann radiation equation provides the total cooling power from a surface at a given temperature. This will all be in the form of radiation in the case of the surface interfaced to vacuum. Due to energy conservation, the radiation resulting when interfaced to an atmosphere will be that total power minus all of the cooling by other competing cooling mechanisms. The alarmists add the other cooling mechanism's power to that of a 100% efficient black body radiator. They then seek a convoluted reason to provide more counteracting warming to this excessive surface cooling in the form of a massive back-radiation.
- At the Earth's surface, the sum of evaporative, conductive, and convective cooling exceed radiative cooling, contrary to the usual alarmist theory.
- A short distance of 100 or 200 meters above the surface, the 65% of the surface infrared radiation that can be absorbed by IR-active gases has been absorbed already due to short mean free path lengths and the energy has been distributed to the non-radiating molecules of the atmosphere due to extremely high collision rates. Only the 35% of surface radiation into the atmospheric window continues on into space under rapid radiative transport. This is 35% of a much smaller amount of surface radiation than posited by the alarmist theory.
- The temperature gradient in the atmosphere near the surface is mostly characterized by slow energy transport mechanisms, not by extremely fast radiative cooling mechanisms imagined by the alarmist theory. Energy transport here is almost entirely upward. Radiation transport is just in very short hops between layers of air usually differing very little in temperature and with few molecules capable of radiating infra-red radiation. This lower part of the troposphere is critically and fortunately not in radiative equilibrium with space.
- Most of the radiation into space is from the upper zone of substantial water vapor concentrations or from still higher altitudes by carbon dioxide. The difference is radiation from the surface into the atmospheric window. The effective temperature of the Earth system as a unitary radiator seen from space is 255K, although only in that it would generate the right amount of total energy as a black body radiator. This temperature is such that it balances the Earth's total absorbed radiation from the sun with an equal cooling radiation into space. This effective black body radiator temperature has no simple connection with the Earth's surface temperature which is the temperature of most importance to human life.
- The gravitational field of the Earth and the Conservation of Energy for static air produce a temperature gradient in the lower atmosphere, the troposphere, which is linear with altitude. In the lowest 5000 m, this decreasing temperature gradient with increasing altitude is about 6.5K/km for dry air. The altitude of effective radiative equilibrium with space at a temperature of 255K is about 5100 meters. Starting from there with a gradient of 6.5K/km produces a temperature at the bottom of the atmosphere of 288K. This matches the average surface temperature.
- The lower atmosphere always has some rising, non-static air due to convection. This rising air expands due to the dropping pressure and cools as it does so. Depending upon the amount of rising convection, the temperature gradient in the troposphere may become as large as 9.78K/km in the bottom 5 km of the atmosphere. The gradient will then be between 6.49 and 9.78K/km depending on the amount of upward air convection. This applies unless winds carry air from areas receiving very different amounts of solar insolation to disturb the area.
- Added carbon dioxide in the alarmist theory causes an increase in back radiation, or in radiation from the Earth's surface being returned to it. But the alarmists overstate the radiation emitted from the Earth's surface by a factor of two and they overstate the radiation returned to the Earth's surface hugely.
- The limited radiation from the Earth's surface that can be absorbed by carbon dioxide is almost entirely absorbed within 100 or 200 meters from the surface. The heat transported by radiation is quickly spread to non-radiating nitrogen and oxygen molecules and to argon atoms that make up 99.97% of the air due to the 6.9 billion collisions per second of molecules. This adds to the slow convective transfer of heat upward.
- Carbon dioxide molecules in the air are rare and will radiate infrared radiation, but it will be at the energy level of the temperature of the surrounding air molecules. Thus they radiate toward the surface as cooler molecules and upward as warmer molecules relative to potential absorbers given the normal temperature gradient in the air with altitude. Consequently, carbon dioxide emitted radiation speeds the transfer of heat toward higher altitudes slightly and only under relatively infrequent conditions can supply the surface with added heat. Carbon dioxide is only about 0.04% of the molecules in the air, placing a limit on the amount of heat transfer at particular wavelengths by so few molecules.
- When the relatively infrequent conditions exist that the emitting carbon dioxide molecules in the air above the surface are warmer than the surface, carbon dioxide emitted radiation is less effectively absorbed by the surface than is that from water vapor. This is because some of the characteristic radiation frequencies of carbon dioxide are not as likely absorbed by water that covers 71% of the planet or by plants based on a water-rich chemistry or by soils and minerals with their commonly high water content.
- Incoming solar radiation is about 49% infrared. Some of this is absorbed in the atmosphere by added carbon dioxide before it can reach the surface and warm it. This results in a cooler surface.
- Carbon dioxide mostly emits radiation into space from altitudes exceeding 9 km and extending to 20 km. From 11 to 20 km there is no temperature change, there being a uniform temperature of about 217K, at least in the U.S. Standard Atmosphere. There may be some temperature change in a tropical atmosphere. There is a version of the carbon dioxide warming theory that more carbon dioxide emitters at this altitude decrease the cooling efficiency of the Earth and that warms the atmosphere below it. Adding carbon dioxide at these altitudes does much less to change the temperature of the emitting molecules since they are already largely emitting in the constant 217K zone. What is more, fast radiative cooling has already become the almost exclusive mode of moving heat to higher altitude and to space due to the water-rich radiation zone at much lower altitudes. More carbon dioxide absorbers at a higher altitude just simply re-emit the radiation quickly into space due to the low gas molecule collision rates. Any radiation directed downward is quickly turned around and also sent into space.
- Even with the considerable very bad physics used to justify a warming effect by carbon dioxide, the warming effect wrongly claimed by the IPCC was only 1.2K upon doubling the amount of carbon dioxide. They then invoked a claimed stronger reinforcing warming due to increased water vapor to make a total warming of 5.4K. Experimental measurements, eons of relatively stable climate, and the expectation of additional cloud cooling and additional solar radiation absorption in the atmosphere due to added water vapor all indicate that increased water vapor would actually provide a negative feedback or a counteracting cooling effect even it added CO2 were to produce a slight warming. Actually, additional CO2 would produce a slight cooling effect.
- The health of plants, upon which we humans and other animals are so dependent, is improved with higher concentrations of carbon dioxide. Carbon dioxide is essential plant food. The improved growth of plants uses up a good portion of any additional carbon dioxide added to the atmosphere.
- Increased infrared active gases tend to moderate the temperature variations of night and day. This is a good thing.
Greenhouse Gas Hypotheses
The physics offered in support
of the hypothesis that IR-absorbing gases are responsible for the large 32.65ºC
temperature difference between the Earth’s calculated effective “black body”
temperature of 255.0K and the average sea level surface temperature of about 287.65K
has some big obstacles to overcome.
Proponents of the hypothesis claim that solar radiation is transmitted
through our atmosphere in the short wavelength portions of the electromagnetic
spectrum as ultra-violet, visible light, and the relatively short wavelength portion
of the infrared radiation dominant in the solar spectrum with little absorption. This radiation is absorbed by the surface of
the Earth and warms it.
The Earth’s surface then emits
long wavelength infrared radiation upward into the atmosphere. The infrared absorbing gases in the
atmosphere absorb most of the IR radiation and re-emit half of it into space and half
of it back toward the surface of the Earth. For this to be true without substantial
energy losses, the time for re-emission of the energy of the absorbed photon
must be very short compared to the time between gas molecule collisions, or some
of the energy will be transferred to other IR-inactive gas molecules. In
addition the mean free path for absorption of an IR-emitter photon before it is
absorbed by an IR-absorber molecule must be large.
Proponents of the catastrophic greenhouse gas hypothesis commonly then claim that the half re-emitted back to the Earth’s surface is then absorbed by the surface and re-emitted toward the atmosphere. A second time the IR-absorbing gases absorb this IR radiation and half of the half is emitted again toward the Earth’s surface. This process repeats infinitely and the net result of adding up all the halves of halves of halves, etc., in a geometric series is said to be about a doubling of the warming power of the solar radiation initially incident upon the surface in the form of back-emitted radiation. Well, this is an interesting violation of energy conservation, so it does not happen. What is more, they assume that the Earth’s surface absorbs all of this re-emitted and returned radiation.
Proponents of the catastrophic greenhouse gas hypothesis commonly then claim that the half re-emitted back to the Earth’s surface is then absorbed by the surface and re-emitted toward the atmosphere. A second time the IR-absorbing gases absorb this IR radiation and half of the half is emitted again toward the Earth’s surface. This process repeats infinitely and the net result of adding up all the halves of halves of halves, etc., in a geometric series is said to be about a doubling of the warming power of the solar radiation initially incident upon the surface in the form of back-emitted radiation. Well, this is an interesting violation of energy conservation, so it does not happen. What is more, they assume that the Earth’s surface absorbs all of this re-emitted and returned radiation.
There is a second, less common,
version of the greenhouse gas warming of the Earth. It basically says that greenhouse gases at
substantial altitudes cool the Earth by radiating energy off into space. The argument then says that adding more of an
IR-absorbing and emitting gas at higher altitudes in the atmosphere will cause
more of the IR-emission to be from cooler molecules as they absorb energy emitted by
IR-active molecules at lower altitudes. They then must disperse some of that energy to the
IR-inactive molecules around them, though this is less likely at these higher altitudes with reduced pressure than it was at lower altitudes. They claim this
decreased cooling at high altitudes causes the lower altitudes and the surface of the Earth to
warm. This argument is often brought forward when the usual high surface emission and high surface absorption of a very high back radiation argument is defeated. The reader might want to think about the inconsistency of this high altitude argument with assumptions made in the back radiation argument. We will talk about this argument
late in this paper as well as another high altitude argument which is totally
inconsistent with this version of the high altitude radiation argument.
IR-Active or Greenhouse Gases
The chemical potential of zero also
causes problems with even thinking you can follow the emissions of individual
photons and count them and figure out how many are absorbed by IR-absorbing
gases and then how many photons are emitted by the excited gas as radiation
versus how much of the energy absorbed by the IR-absorbing gas is lost due to
collisions with the many other gas molecules in the lower atmosphere. This is a real problem, since below about
4000 meters altitude, more energy is transferred by collisions, mostly to
nitrogen and oxygen molecules, than is transferred by radiation. To further complicate things, energy is also
transported by the evaporation of water, the sublimation of ice, the
condensation of water vapor, by air conduction and convection currents, by
winds, and by the expansion of warm air as it rises. These other energy transfer mechanisms are
the reason why the Earth’s surface itself is not in thermal radiative
equilibrium with space as the sphere at the 5,100 meter altitude effectively is.
Heat Capacity of the Surface Effects
Analogously,
the Earth's land surface, its oceans covering 70% of the planet, and its
atmosphere all have a heat capacity and provide for a substantial flow of heat to
the surface from their interiors at various times of the day. The heat capacities of the Earth’s surface
and atmosphere greatly exceed that of the rock of the moon, especially thanks
to our oceans, so the day to night moderating effect seen on the Earth is much
larger than it is for the moon. It is true, as pointed out by Rosco in a comment, that the moon also achieves both higher and lower temperatures than would the Earth simply because its daily cycle is much longer. Nonetheless, the radiative equivalent temperature of the Earth as a whole and averaged over the day would be lower than 255K if the solar insolation reaching the surface were unchanged because higher daylight temperatures would cause more effective cooling during the day than during the night.
The size of the effect of the ocean is found to be most dramatic for small islands surrounded by ocean in the equatorial area in which the day to night temperature shift is very small. This much more moderate difference in the day and night surface temperatures results in a much lower effective increase in the surface temperature than the 40K increase seen on the moon due to differences in the radiative cooling between day and night.
But with the fairly typical 22ºF high to low temperature difference at the mid-latitude Baltimore-Washington International Airport averaged over a year, the radiative cooling at the daily high temperature is about 18.5% more efficient than the radiative cooling at the daily low temperature. We also have to remember that like the moon, we have an underlying warming effect due to the sub-surface storage of energy at night and the cooler sub-surface during the day. The extreme moderation of the Earth’s daily cycle is also the only reason we can even do baseline calculations at all using a daily average set of conditions without huge errors. We should remember that this is still a crude approximation and that we are making it still cruder by ignoring the wider differences in radiative cooling between the Equator and the Poles.
The Black Body and the Earth Radiator
IR-Active or Greenhouse Gases
All IR-absorbing
gases do is capture energy for an instant due to the vibrational excitation of their chemical bonds or due to inducing electronic transitions and then they release it, either by
radiating it away or by colliding with another gas molecule such as the
predominant nitrogen or oxygen molecules and transferring energy to them. These predominant molecules of nitrogen and
oxygen then transfer this collision-absorbed energy through convection and gas
collisions with other molecules. But,
none of these effects do more than transfer energy. They do not create it. They do not magnify the energy of the sun or
serve as a supplementary source of energy, though the evaporation and
condensation of water do greatly affect the distribution of energy in the
atmosphere. There is no analog to these many water vapor roles
for the much rarer CO2, methane, or nitrous oxide. In addition, the lifetime of methane and
nitrous oxide is shorter since they are broken down by UV radiation.
Another basic
reason the greenhouse gas or IR-absorbing gas idea of emitted, half re-absorbed,
and then re-emitted, then half re-absorbed once again in a geometric power series does not work is because the photons of
radiation inside a black body radiator do not behave like ordinary
particles. They are bosons and radiation
from the walls of the black body varies to keep the conditions on the hollow
interior of a black body sphere at constant temperature in equilibrium. The energy density per unit interior volume remains
constant for a given temperature inside the black body sphere even if you
expand the sphere and make it bigger. To keep that constant energy density per
unit volume, the walls actually produce more photons per unit area when you
make the sphere volume larger. This
larger flux of photons off both the inner wall and outer wall surfaces
corresponds to the same black body temperature.
Doubling the radius of the black body sphere of a given temperature
causes the flux of photons per unit surface area to also double. This is not very intuitive for most
people. Indeed, it is not intuitive to
most people who have long studied physics.
You cannot in a similar way increase the number of atoms, for instance.
The chemical potential of black-body radiation is zero, which is a most remarkable property. This can contribute to many misunderstandings of how black body radiation is to be applied to real-world objects. It also is important in understanding why a warmer body does not generally absorb radiation from a cooler body, despite a flux of photons from the cooler body being incident upon the warmer body. Due to local fluctuations and to the Boltzmann velocity distribution of gas molecules there are some exceptions of absorption in the Earth's surface of a photon emitted from somewhat cooler air above it, but this is a very insignificant effect.
The chemical potential of black-body radiation is zero, which is a most remarkable property. This can contribute to many misunderstandings of how black body radiation is to be applied to real-world objects. It also is important in understanding why a warmer body does not generally absorb radiation from a cooler body, despite a flux of photons from the cooler body being incident upon the warmer body. Due to local fluctuations and to the Boltzmann velocity distribution of gas molecules there are some exceptions of absorption in the Earth's surface of a photon emitted from somewhat cooler air above it, but this is a very insignificant effect.
Heat Capacity of the Surface Effects
A very
interesting article by Martin Hertzberg, Hans Schreuder, and Alan Siddons
called A Greenhouse Effect on the Moon?, should be summarized here and
discussed in this context. The moon has
no atmosphere and it is the same distance from the sun as the Earth is. Yet, the mid-day temperature on the moon's
surface is about 370K or about 97º C, which is about 20K cooler than expected
just due to the radiation incident from the sun. The nighttime temperature gets down to about
85K or about -188º C, but this is about 60K warmer than the expected low
temperature due to radiative cooling as the only nighttime energy flow. See the daily temperature profile in the
figure below comparing the predicted temperature with the actual
temperature.
The reason for the difference is that the surface of the moon holds and retains heat into its night due to its heat capacity and the sub-surface remains somewhat cooler than the immediate surface during its day. The subsurface rock cools the surface then. These effects make the average temperature of the moon’s surface about 228K. This is about 40K warmer than it would otherwise be due to reduced radiative cooling during the day and increased radiative cooling during the night. The night cooling is at a much less cooling-efficient lower temperature than the day temperature. This increase of average temperature over the daily cycle owes to the fourth power dependence of radiative cooling on the temperature and the large daily swing in the temperature.
The reason for the difference is that the surface of the moon holds and retains heat into its night due to its heat capacity and the sub-surface remains somewhat cooler than the immediate surface during its day. The subsurface rock cools the surface then. These effects make the average temperature of the moon’s surface about 228K. This is about 40K warmer than it would otherwise be due to reduced radiative cooling during the day and increased radiative cooling during the night. The night cooling is at a much less cooling-efficient lower temperature than the day temperature. This increase of average temperature over the daily cycle owes to the fourth power dependence of radiative cooling on the temperature and the large daily swing in the temperature.
Fig. 1. The predicted temperature of the moon’s
surface with no ground thermal conductivity and sub-surface heat capacity
compared to the real measured temperatures. The blue line shows the expected
temperature if the subsurface heat capacity did not play the moderating role it
does.
The size of the effect of the ocean is found to be most dramatic for small islands surrounded by ocean in the equatorial area in which the day to night temperature shift is very small. This much more moderate difference in the day and night surface temperatures results in a much lower effective increase in the surface temperature than the 40K increase seen on the moon due to differences in the radiative cooling between day and night.
But with the fairly typical 22ºF high to low temperature difference at the mid-latitude Baltimore-Washington International Airport averaged over a year, the radiative cooling at the daily high temperature is about 18.5% more efficient than the radiative cooling at the daily low temperature. We also have to remember that like the moon, we have an underlying warming effect due to the sub-surface storage of energy at night and the cooler sub-surface during the day. The extreme moderation of the Earth’s daily cycle is also the only reason we can even do baseline calculations at all using a daily average set of conditions without huge errors. We should remember that this is still a crude approximation and that we are making it still cruder by ignoring the wider differences in radiative cooling between the Equator and the Poles.
The Black Body and the Earth Radiator
Let us examine some of the
properties of black body radiation for a moment. The power in Watts (W) radiated by a black
body surface at a temperature T (in Kelvin) into vacuum is given by the
Stefan-Boltzmann Law formula:
P
= A ε σ T4,
in which A is
the radiating area in square meters, σ = 5.6697 x 10-8 W/m2K4
the Stefan-Boltzmann constant, and ε = 1.
A watt is equal to a joule/second, or J/s, and a joule is a unit of energy. Heat is energy.
The area of a sphere of radius r is 4 π r2. The altitude of 5000 meters above sea level according to the temperatures of the U.S. Standard Atmosphere of 1976 is 255.7 K, which is almost equal to the Earth’s effective black body radiation temperature as seen from space, which is about 255K. The altitude actually at 255K is about 5105 m. By this it is only meant that a black body radiator at the temperature of 255K would radiate the same total amount of energy as the Earth does. The Earth’s radius is about 6,376,000 meters, so the effective sphere that is in equivalent radiant equilibrium with space has a radius slightly larger of about 6,381,100 meters. If this sphere’s surface were uniformly at the temperature of 255K, then its total radiant outward power would be 1.227 x 1017 W. That sphere would also emit a total inward radiant power of the same amount and all inside the shell wall of the sphere would be in equilibrium, were it not for our atmosphere.
The area of a sphere of radius r is 4 π r2. The altitude of 5000 meters above sea level according to the temperatures of the U.S. Standard Atmosphere of 1976 is 255.7 K, which is almost equal to the Earth’s effective black body radiation temperature as seen from space, which is about 255K. The altitude actually at 255K is about 5105 m. By this it is only meant that a black body radiator at the temperature of 255K would radiate the same total amount of energy as the Earth does. The Earth’s radius is about 6,376,000 meters, so the effective sphere that is in equivalent radiant equilibrium with space has a radius slightly larger of about 6,381,100 meters. If this sphere’s surface were uniformly at the temperature of 255K, then its total radiant outward power would be 1.227 x 1017 W. That sphere would also emit a total inward radiant power of the same amount and all inside the shell wall of the sphere would be in equilibrium, were it not for our atmosphere.
If we assume
that the sphere with the temperature of 255K is in equilibrium with a slightly
smaller black body sphere of the radius of the Earth at sea level, we can
calculate the temperature of that surface given that it must radiate a power
equal to the power of the surrounding sphere which is in equilibrium with
space. The temperature will be higher,
since the surface area of the sphere is smaller. In fact, the temperature of the Earth’s
surface as a black body would be 255.100K or 0.1ºC warmer than the sphere at
the altitude of 5100 meters above sea level which is in equilibrium with space
in this very simple model. Thus we see
that the altitude itself of the radiating surface, whether the Earth’s surface
or the weighted average altitude in the atmosphere makes no significant
difference from the standpoint of the size of the radiating surface.
But the
Earth is not really a black body, so the Stefan-Boltzmann equation
has to have an emissivity factor, ԑ, multiplied times the temperature side of
the equation. For the Earth as a whole, this
emissivity factor is often said to be about 0.7. This is an effective
emissivity of the Earth’s surface and the various altitudes of its atmosphere combined
in some unknown weighted average. This
causes the Earth’s effective radiative altitude or plane to have to be at the more
elevated temperature of 278.9K to be in equilibrium. This effective sphere with this temperature is somewhere between the Earth's surface and altitudes from which most atmospheric radiation into space occurs. The effective plane of radiation according to the U.S. Standard Atmosphere with this temperature is at 1433 m altitude. Thus it is reasonable that the temperature we calculate here is between that of the surface and the cooler altitude from which much of the atmospheric radiation is emitted. This plane is only about 8.75K or 8.75ºC below the surface
temperature of 287.65K. Thus, the effective radiative plane temperature differs from the surface temperature by only 26.8% of the 32.65K claimed greenhouse gas contribution. The surface temperature is warmer than this, as it should be, and there is no great temperature discrepancy to be explained.
Of course, the sphere around the Earth with a radius 5,100 meters greater than that of sea level is not really at a constant temperature, since part of the Earth is in daylight and part is in nighttime. Nonetheless, the above calculation gives us a good sense of the magnitude of real radiant effects by black body (ε=1) and gray body (ε less than 1) radiators because for Earth the day and night temperatures are not terribly different, given the wondrous effect of its very high heat capacity near the surface. The gray body calculation makes it very clear that any IR-absorbing gas effects that do exist do not necessarily provide a 32.65º C increase of the surface temperature in the way in which that is usually described by alarmist propaganda.
There are many
issues of interest that remain to be examined in much more detail. If the Earth’s surface were in radiative
thermal equilibrium with the atmosphere at an altitude of 5100 m, its
temperature would be (255 + 0.1)K = 255.1K as we calculated above. One critical issue is that the Earth’s surface
is not in radiative equilibrium with the sphere at about 5100 meters above it
for more reasons even than the evaporation and condensation of water and air
conduction and convection. The ground or
the surfaces of the oceans with their high heat capacities retain heat obtained
during the daytime into the night. Also, the temperature at the surface and
even at an altitude of 5,100 meters is certainly a function of how much of the
solar radiation ever reaches as deep into our atmosphere as the lower few thousand
meters and to sea level. If the
atmosphere were to absorb more radiation in the UV, visible, and IR spectrum of
the incoming solar radiation, that would cool the Earth’s surface. More of the heat from the sun might be
retained higher in the atmosphere.
Of course, the sphere around the Earth with a radius 5,100 meters greater than that of sea level is not really at a constant temperature, since part of the Earth is in daylight and part is in nighttime. Nonetheless, the above calculation gives us a good sense of the magnitude of real radiant effects by black body (ε=1) and gray body (ε less than 1) radiators because for Earth the day and night temperatures are not terribly different, given the wondrous effect of its very high heat capacity near the surface. The gray body calculation makes it very clear that any IR-absorbing gas effects that do exist do not necessarily provide a 32.65º C increase of the surface temperature in the way in which that is usually described by alarmist propaganda.
The widely used 1997 version of the Kiehl-Trenberth energy budget for the Earth is given in Fig. 2 below. This energy budget was featured in the UN IPCC 4th report of 2007. The right-hand side and center of this diagram showing surface cooling effects and back-radiation is total nonsense, while the left side showing solar insolation and the effects upon it, is not so far from the truth. According to this diagram, about 198 W/m2 of solar insolation reaches the surface, but about 15.2% of that is reflected. It is probably more realistic that 64% of the solar insolation is incident upon the surface, which is 219 W/m2, and if 15.2% of that is reflected, then the surface absorbs about 186 W/m2 with about 33 W/m2 reflected from the surface. The radiative cooling potential of a surface into vacuum absorbing an influx of power of 186 W/m2 at a temperature of 14.5ºC, or 287.65K, implies that
P
= 186 W/m2 = ԑ σ T4 = ԑ (5.6697 x 10-8 W/m2K4)(287.65
K)4,
Where ԑ is
the emissivity of the Earth’s surface, which implies ԑ = 0.479.
Performing the same calculation using the K-T diagram absorbed solar insolation at the Earth's surface yields a lower bound emissivity of 0.433. This is the lower bound because it assumes that the solar insolation absorbed by the atmosphere is not re-radiated to the Earth's surface and absorbed there. Actually, it is not really even a lower bound effectively because we are also assuming here that the Earth's surface has no other mechanisms for losing heat. We are explicitly ignoring the evaporation of water, conduction, and convection currents!
We can obtain an upper bound emissivity for the Earth's surface as well. Let us be very generous and assume that half of the incoming solar flux absorbed by the atmosphere is re-emitted toward the surface and half toward space. The highest energy flux that could be absorbed by the surface would then be the direct 168 W/m2 directly absorbed according to K-T and half of the 67 W/m2 they claim was initially absorbed by the atmosphere. This very generous upper bound of 201.5 W/m2 would mean that the emissivity into space was 0.519. Note that this is the emissivity of the surface of the Earth, which is different from the weighted average of the Earth’s surface and the atmosphere at altitude in radiative equilibrium with space, which we said earlier had an effective ε about 0.7.
So, the K-T diagram implies that the Earth's surface emissivity lies between about 0.43 and 0.52 if the Earth were in equilibrium with vacuum. The source of energy flux into the Earth's surface is the energy from the sun, ignoring the very minor contribution from the Earth's hot interior. So, if the Earth's surface interfaced to vacuum, it would have to have an emissivity of about 0.48 to equilibrate the energy flux into the surface with that emitted from it at a temperature of 287.65K. But because other energy transport mechanisms are at work at the interface, the equation will only provide us with the total energy transported across the interface. That energy will now be such that the sum of all such energy transport fluxes will equal about 186 W/m2 to use my preferred value between the direct solar insolation of 168 W/m2 and the upper bound of maximum solar power possible obtained by adding in half the solar insolation absorbed by the atmosphere giving 201.5 W/m2. The emissivity is then about 0.48, which hugely bothers the many climate scientists who claim the emissivity is about 0.95 or maybe 0.93.
There is no way to conserve the input energy from the sun and arrive at an effective surface emissivity for the Earth's surface of 0.95. Near the end of this paper, I will present many infra-red absorption spectra of common materials found at the Earth's surface and it will be readily observable that the absorptivity is not close to 0.95 for any of the materials. This makes it very unlikely that their emissivity is close to 0.95 either.
There is still another way in which the emissivity here is an effective value. While the temperature we associate with the surface is 287.65K, the very thin layer of the last few nanometers of material before the interface with the air is cooler due to water evaporation from that surface and through much of the day due to cooler air molecule collisions with the surface. Thus the surface emission radiation is actually going to be suppressed by this cooler temperature immediately at the surface due to limited thermal conduction of materials, but the total energy transport across this thin layer must be the same whether the atmosphere causes this or not. When using the supposed warmer temperature of that surface, one winds up compensating by calculating too low an emissivity. Consequently, this calculated Earth emissivity above is an effective emissivity.
It is not surprising that it is lower than the emissivity claimed for water in the IR wavelengths of 0.95 to 0.98. Those water emissivity measurements are very hard to make and may be unreliable in any case. It is clear that water is not a black body like absorber of IR radiation as we will see later. That being the case, it is surprising that it is claimed to be a near black body emitter. According to Kirchoff's Law, the emissivity and the absorptivity are equal. In truth, they need not be equal for gray body radiators. Water is actually relatively transparent to infra-red at many wavelengths, though the absorption, as seen later in Fig. 7. is never zero below 3700 cm-1, so complete absorption may take many meters of depth below the surface. Most of the Earth's surface is covered with highly impure ocean water with many particulates suspended in it and these are scatters that may scatter infra-red radiation back to the atmosphere.
More important, the solar insolation absorbed a meter below the surface is absorbed into a layer of water that is cooler than the air an equal distance or even several times the distance above the water. This means that there is no radiative transfer of heat from that cooler water layer to the air above the water. Now for those infra-red frequencies where the emissivity of water is high, water vapor above the surface of the water can absorb the emitted infra-red, provided that the water vapor absorber is at a lower temperature than the water molecule at a depth below the surface. But the common mean free path for water absorption is so short in the several meters above water surfaces at these frequencies that this condition is not often met. On the other hand, liquid water will emit at frequencies which water vapor cannot absorb, so the lower probability emission events at these frequencies can travel through the atmospheric window and so a low level of radiation from beneath the surface layer of water may occur. The end result is that despite the apparent high absorptivity of the water due to the great absorption depth of most bodies of water, the effective emissivity is much lower than the apparent total absorptivity.
A reasonable estimate of the potential surface emissivity is then ԑ = 0.5. I am using the “potential” qualifier, because any other cooling mechanism reduces this radiative cooling. Therefore, this is really an upper bound on the effective ε value and the radiative cooling.
Performing the same calculation using the K-T diagram absorbed solar insolation at the Earth's surface yields a lower bound emissivity of 0.433. This is the lower bound because it assumes that the solar insolation absorbed by the atmosphere is not re-radiated to the Earth's surface and absorbed there. Actually, it is not really even a lower bound effectively because we are also assuming here that the Earth's surface has no other mechanisms for losing heat. We are explicitly ignoring the evaporation of water, conduction, and convection currents!
We can obtain an upper bound emissivity for the Earth's surface as well. Let us be very generous and assume that half of the incoming solar flux absorbed by the atmosphere is re-emitted toward the surface and half toward space. The highest energy flux that could be absorbed by the surface would then be the direct 168 W/m2 directly absorbed according to K-T and half of the 67 W/m2 they claim was initially absorbed by the atmosphere. This very generous upper bound of 201.5 W/m2 would mean that the emissivity into space was 0.519. Note that this is the emissivity of the surface of the Earth, which is different from the weighted average of the Earth’s surface and the atmosphere at altitude in radiative equilibrium with space, which we said earlier had an effective ε about 0.7.
So, the K-T diagram implies that the Earth's surface emissivity lies between about 0.43 and 0.52 if the Earth were in equilibrium with vacuum. The source of energy flux into the Earth's surface is the energy from the sun, ignoring the very minor contribution from the Earth's hot interior. So, if the Earth's surface interfaced to vacuum, it would have to have an emissivity of about 0.48 to equilibrate the energy flux into the surface with that emitted from it at a temperature of 287.65K. But because other energy transport mechanisms are at work at the interface, the equation will only provide us with the total energy transported across the interface. That energy will now be such that the sum of all such energy transport fluxes will equal about 186 W/m2 to use my preferred value between the direct solar insolation of 168 W/m2 and the upper bound of maximum solar power possible obtained by adding in half the solar insolation absorbed by the atmosphere giving 201.5 W/m2. The emissivity is then about 0.48, which hugely bothers the many climate scientists who claim the emissivity is about 0.95 or maybe 0.93.
There is no way to conserve the input energy from the sun and arrive at an effective surface emissivity for the Earth's surface of 0.95. Near the end of this paper, I will present many infra-red absorption spectra of common materials found at the Earth's surface and it will be readily observable that the absorptivity is not close to 0.95 for any of the materials. This makes it very unlikely that their emissivity is close to 0.95 either.
There is still another way in which the emissivity here is an effective value. While the temperature we associate with the surface is 287.65K, the very thin layer of the last few nanometers of material before the interface with the air is cooler due to water evaporation from that surface and through much of the day due to cooler air molecule collisions with the surface. Thus the surface emission radiation is actually going to be suppressed by this cooler temperature immediately at the surface due to limited thermal conduction of materials, but the total energy transport across this thin layer must be the same whether the atmosphere causes this or not. When using the supposed warmer temperature of that surface, one winds up compensating by calculating too low an emissivity. Consequently, this calculated Earth emissivity above is an effective emissivity.
It is not surprising that it is lower than the emissivity claimed for water in the IR wavelengths of 0.95 to 0.98. Those water emissivity measurements are very hard to make and may be unreliable in any case. It is clear that water is not a black body like absorber of IR radiation as we will see later. That being the case, it is surprising that it is claimed to be a near black body emitter. According to Kirchoff's Law, the emissivity and the absorptivity are equal. In truth, they need not be equal for gray body radiators. Water is actually relatively transparent to infra-red at many wavelengths, though the absorption, as seen later in Fig. 7. is never zero below 3700 cm-1, so complete absorption may take many meters of depth below the surface. Most of the Earth's surface is covered with highly impure ocean water with many particulates suspended in it and these are scatters that may scatter infra-red radiation back to the atmosphere.
More important, the solar insolation absorbed a meter below the surface is absorbed into a layer of water that is cooler than the air an equal distance or even several times the distance above the water. This means that there is no radiative transfer of heat from that cooler water layer to the air above the water. Now for those infra-red frequencies where the emissivity of water is high, water vapor above the surface of the water can absorb the emitted infra-red, provided that the water vapor absorber is at a lower temperature than the water molecule at a depth below the surface. But the common mean free path for water absorption is so short in the several meters above water surfaces at these frequencies that this condition is not often met. On the other hand, liquid water will emit at frequencies which water vapor cannot absorb, so the lower probability emission events at these frequencies can travel through the atmospheric window and so a low level of radiation from beneath the surface layer of water may occur. The end result is that despite the apparent high absorptivity of the water due to the great absorption depth of most bodies of water, the effective emissivity is much lower than the apparent total absorptivity.
A reasonable estimate of the potential surface emissivity is then ԑ = 0.5. I am using the “potential” qualifier, because any other cooling mechanism reduces this radiative cooling. Therefore, this is really an upper bound on the effective ε value and the radiative cooling.
Fig. 2. Kiehl-Trenberth energy budget for the Earth of 1997. This represents a common viewpoint of the physics that is used to justify the catastrophic man-made global warming hypothesis. It is apparently the settled science. It will be demonstrated to be very wrongheaded. I have added the percentage power flux values with 342 W/m2 equal to 100% and approximately equal to one-quarter of the solar power incident upon the Earth most directly facing the Sun.
Let us use the
calculated Earth surface emissivity of 0.5 then to calculate the upper limit on
the surface radiation for comparison to the Kiehl-Trenberth diagram where it is
said to be 390 W/m2, a power flux even larger than the 342 W/m2
incident at the top of the atmosphere as an average over the daily cycle. What we find is that the surface emits no
more than 195 W/m2, which is half of the Kiehl-Trenberth surface
radiation since they assumed ԑ = 1. Let
me repeat that this is an upper bound. The
actual radiative cooling of the surface is much less due to heat loss by
air conduction, air convection, and water evaporation and other cooling
mechanisms.
Discussion of the Energy Balance in the Kiehl-Trenberth Energy Budget
Discussion of the Energy Balance in the Kiehl-Trenberth Energy Budget
The
consequences of this are huge. Because
the catastrophic man-made global warming theory posits a huge radiative cooling of the surface
due to a large radiative power flux back to the atmosphere, it is
forced to conjure up some mechanism whereby a very large fraction of this power
can be returned to the surface. Their
large value of back radiation is 324 W/m2, which is 83% of the radiative cooling of a
black body surface due to its being at the temperature of 288K! Yet each time a greenhouse gas absorbs
radiation in a limited frequency range in which it can do so, it sends half the
power off toward space and cannot return more than the other half toward the
surface! Let us assume that half of all
the power inputs into the atmosphere in their diagram are returned as absorbed
IR radiation to the ground. The maximum
value of back radiation would then be (0.5) (350 + 67 + 24 + 78) = 260 W/m2,
not 324 W/m2. Yet even this
is too high to be a proper upper limit, since the potential ground radiated
power is only about 195 W/m2.
There is
another problem here as well. If half of
the radiation in the atmosphere is returned to the surface and half is emitted
into space and they claim that 324 W/m2 is returned to the surface,
where is the 324 W/m2 which is emitted into space? In fact one should have the 40 W/m2
emitted from the surface through the atmospheric window without absorption
added to the supposed 324 W/m2 emitted from the atmosphere after being
absorbed there and to the 30 W/m2 which appears to be due to the heat
of condensation of water in clouds causing cloud tops to emit IR into
space. The sum of these quantities would
be 394 W/m2. This greatly exceeds the original incoming insolation
of 342 W/m2 minus the reflected portion of that which is 107 W/m2, for a remainder of only 235 W/m2.
The energy flux into space should be 394 W/m2 then compared to absorbed solar insolation of only 235 W/m2. There is no real energy balance here. They just absorbed 350 W/m2 of surface emitted IR radiation in the atmosphere and arbitrarily added only 165 W/m2 of IR-emitted energy from the atmosphere into space to the 40 W/m2 from the surface through the atmospheric window and to the 30 W/m2 from condensation of water in clouds. These numbers just appeared to be jiggered to provide apparent power flux conservation for solar insolation with the radiation of the Earth as a whole into space and to provide the right sum of power flux numbers into the atmosphere and into the surface, but without actually providing total consistency and total power balance.
It is also interesting to note that the 78 W/m2 of evaporative cooling of the surface is not matched by the heat generated in clouds when that same water condenses to produce the heat of condensation! Of course that remaining heat due to condensation could fall to the surface as warm rain, but where is that in the diagram? It turns out that they added all of that power to help generate a large back radiation component.
The energy flux into space should be 394 W/m2 then compared to absorbed solar insolation of only 235 W/m2. There is no real energy balance here. They just absorbed 350 W/m2 of surface emitted IR radiation in the atmosphere and arbitrarily added only 165 W/m2 of IR-emitted energy from the atmosphere into space to the 40 W/m2 from the surface through the atmospheric window and to the 30 W/m2 from condensation of water in clouds. These numbers just appeared to be jiggered to provide apparent power flux conservation for solar insolation with the radiation of the Earth as a whole into space and to provide the right sum of power flux numbers into the atmosphere and into the surface, but without actually providing total consistency and total power balance.
It is also interesting to note that the 78 W/m2 of evaporative cooling of the surface is not matched by the heat generated in clouds when that same water condenses to produce the heat of condensation! Of course that remaining heat due to condensation could fall to the surface as warm rain, but where is that in the diagram? It turns out that they added all of that power to help generate a large back radiation component.
I have noted
before that a very critical aspect of our atmosphere is that the lower
troposphere part of the atmosphere disrupts the thermal radiative equilibrium between the surface and
space. It is well up into the atmosphere
at an effective altitude of about 5.1 km that radiation significantly dominates and other heat
transport mechanisms become significantly less important than radiation. Yet, the K-T diagram inverts this
relationship and claims that radiation fluxes in the lower atmosphere dominate
all other energy transport mechanisms and actually transport larger amounts of
energy back and forth by far between the lower troposphere and the surface than
they do in the upper atmosphere and back into space. This is an incredible distortion of the
reality.
Let us return
to the difference in the radiative cooling due to the daily high and low
temperatures. Radiative cooling was
18.5% more efficient at the daily high of about 294.25 K at mid-latitude BWI
Airport than it was at the daily low average of about 282.05 K. Radiative cooling at the daily average high
is about 8.8% more efficient than at the average temperature, while radiative
cooling at the daily low is about 8.2 % less efficient than at the average
temperature. The daily cycle radiative
cooling boost for Earth based on a simple average of these high and low cooling
efficiencies is only an increase in average radiative cooling efficiency of
0.3% compared to that at the daily average.
For most purposes in our discussion to follow, this is a small effect
and can be ignored. It is not so small
when one begins to discuss the effects of increasing the concentration of CO2
in the atmosphere, however. For now we
will note that 195 W/m2 of surface radiative cooling we calculated
above based on the average temperature may really be more like 196 W/m2,
so we will henceforth take 196 W/m2 as a more accurate daily average.
The Temperature Gradient in the Troposphere Due to Gravity and that due to Convection
The Temperature Gradient in the Troposphere Due to Gravity and that due to Convection
Let
us also recall that there is a warming effect caused by gravity acting on the
gas molecules of the atmosphere between the altitude in the atmosphere in
radiative equilibrium with space and the Earth’s surface, which is not in
radiative equilibrium. This is because
the potential energy of a gas molecule at 5000 meters altitude added to its
kinetic energy equals the kinetic energy at sea level, assuming we set the
potential energy to zero at sea level.
Then there is a linear gradient in kinetic energy with altitude. The temperature of a perfect gas molecule is
proportional to its kinetic energy, so an increased kinetic energy at sea level
compared to its kinetic energy at 5000 meters altitude means the gas molecule
is warmer at sea level.
EK = (3/2) kT, where EK is the kinetic energy for a perfect monatomic gas molecule, where k is the Boltzmann constant. However, the lower atmosphere is made up almost entirely of diatomic molecules, with N2 and O2 more than 99% of the atmosphere. EK = (5/2) kT for a diatomic perfect or ideal gas molecule and (6/2)kT for a polyatomic molecule with more than two atoms. This is because a diatomic molecule has rotational kinetic energy around each axis perpendicular to the bond between the two atoms in the molecule. There are equal amounts of energy in each of the 5 degrees of freedom of the diatomic molecule. Molecules such as CO2 and CH4 with more than two atoms have 6 degrees of kinetic energy freedom. This allows us to tie the total kinetic energy at an altitude to the translational velocities of molecules given in the U.S. Standard Atmosphere table of 1976 for dry air. The total kinetic energy of the diatomic molecules making up more than 99% of the lower atmosphere is then 5/3 times the translational kinetic energy.
EK = (3/2) kT, where EK is the kinetic energy for a perfect monatomic gas molecule, where k is the Boltzmann constant. However, the lower atmosphere is made up almost entirely of diatomic molecules, with N2 and O2 more than 99% of the atmosphere. EK = (5/2) kT for a diatomic perfect or ideal gas molecule and (6/2)kT for a polyatomic molecule with more than two atoms. This is because a diatomic molecule has rotational kinetic energy around each axis perpendicular to the bond between the two atoms in the molecule. There are equal amounts of energy in each of the 5 degrees of freedom of the diatomic molecule. Molecules such as CO2 and CH4 with more than two atoms have 6 degrees of kinetic energy freedom. This allows us to tie the total kinetic energy at an altitude to the translational velocities of molecules given in the U.S. Standard Atmosphere table of 1976 for dry air. The total kinetic energy of the diatomic molecules making up more than 99% of the lower atmosphere is then 5/3 times the translational kinetic energy.
Conservation
of energy for a diatomic gas molecule requires that:
EK0
= (5/3) (½ m v02 ) = EK5000 = (5/3)(½ m v50002
) + mgh,
Where EK0 is the energy of the gas molecule at sea level, v0 is its translational velocity there, EK5000 is the energy at 5000 meters altitude, v5000 is the translational velocity of the gas molecule at 5000 meters altitude, m is the mass of the molecule, g is the gravitational constant at 5000 meters altitude, and h is the altitude, here 5000 m. From the U.S. Standard Atmosphere table of 1976, the mean gas molecule in the atmosphere has a mass of 28.964 amu or 4.8080 x 10-26 kg, which is greater than the mass of the most common N2 molecules and lower than the mass of the second most common O2 molecules. The gravitational constant at 5000 meters altitude is slightly less than that at sea level and is found in the table to be 9.7912 m/s2. The translational velocity of the mean molecule at 5000 meters altitude from the table is 432.31 m/s. From this, we calculate that v0 is 495.62 m/s. The U.S. Standard Atmosphere sea level velocity is 458.94 m/s, implying that other effects are providing significant cooling of the atmosphere at sea level. The value of EK0 is calculated to be 9.8419 x 10-21 Joules per mean molecular weight air molecule at sea level.
We can now set the gravitational effect EK0 kinetic energy into the EK = (5/2) kT equation and calculate what T should be if there were no other cooling effects, such as the evaporation of water. Note that air convection is not a net changer of the energy here, except for the effect of volume expansion cooling as the warm air rises and the pressure drops. The temperature gradient exists in the static air, yet there is no flow of heat. We find that the surface of the Earth, at sea level, should have a temperature of 285.07K, or 11.92ºC, or 53.46ºF, which is 30.1K warmer than the 255K it would have if the surface itself were in direct radiative equilibrium with space as a black body, assuming a nearly constant temperature throughout a day. Of course the Earth is not a black body as we discovered and with an emissivity of 0.5 and an absorbed solar insolation of 186 W/m2, the expected surface temperature is 284.61K, or about the same temperature as is expected given its thermal equilibrium with the bottom of the atmosphere at 285.07K. Thus the bottom of the atmosphere expected temperature due to the static equilibrium gravitational field effect is only 2.58K less than the commonly quoted average surface temperature of the Earth and the Earth’s surface itself is only 3.04K less than the average surface temperature.
From the U.S. Standard Atmosphere table of1976 for dry air, the temperature at 5 km altitude is 255.68K. If the surface temperature were 285.07K, the effective lapse rate per 1 km elevation between 5 km and sea level would be 5.88K/km. Weighting monatomic, diatomic, and polyatomic molecules for the relationship of their total kinetic energy to their translational kinetic energy and weighting the total kinetic energy relation to the temperature, the calculated static gravitational gradient increases slightly to 5.93K/km. Using this gradient, the surface temperature would be 285.33K. This still has errors due to treating each molecule as having the mean weight and mean velocity. Of course the surface temperature is slightly higher at 288.15K, so the static equilibrium gravitational gradient is really 6.49K/km. This difference between 5.93K/km and 6.49K/km is not due to water vapor in static air. Water vapor has a large effect upon the dynamic adiabatic lapse rate, but a small effect upon this static equilibrium temperature gradient due to gravity alone. Adding water decreases the mean molecular weight and increases the fraction of molecules with 6 degrees of freedom, but there is so little water usually that the effect on this temperature gradient is still small.
At this point, one might ask if the U.S. Standard Atmosphere table of 1976 is consistent with the ideal gas law of PV = nRT? It is. If we examine the case for 1 m3 of air at sea level and for the same volume at 5000 m altitude, we have
T5000 / T0 = (n0P5000)
/ (n5000P0) = (δ0P5000) /
(δ5000P0),
where δ is the density of the atmosphere at the given altitude. The table provides δ0 = 1.2250 kg/m3, δ5000 = 0.73643 kg/m3, P0 = 1013.25 mb, and P5000 = 540.48 mb, with mb being millibars. The table provides the surface temperature at sea level as 288.15K, and the ratio formula above then says the temperature T5000 = 255.674, in agreement with the table value given as 255.676K. The fact that the molecule energy conservation formula used above that yielded a surface temperature of 285.07K was slightly different than 288.15K is the measure to which the air does not represent quite a perfect and ideal gas primarily, but secondarily to the neglect of the slightly less than 1% of gases which are almost entirely monatomic molecules and have only translational kinetic energy. The neglect of the monatomic gases would have dropped the surface temperature slightly, though most of this difference is due to a small deviation of air from being a perfect gas.
The theoretical thermodynamic derivation of the gravitational temperature gradient along an adiabatic pathway is commonly given to be g/Cp after a correction to a derivation by Loschmidt in the 19th century, where g is the gravitational “constant”, varying from 9.8066 to 9.7912 m/s2 between sea level and 5 km altitude. Cp is the heat capacity at constant pressure of dry air, which between 250K and 300K increases from 1.003 to 1.005 KJ/kgK. Consequently, the lapse rate calculated from the g/Cp formula is 9.76K/Km. If we applied that lapse rate to calculate the Earth’s surface temperature with respect to the approximately radiative equilibrium temperature at 5 km of 255.68K, we would have a higher average surface temperature of 304.7K, which is 16.5K warmer than the actual surface temperature.
Consequently, we can conclude that the prediction of a lapse rate of g/Cp is not applicable to the atmosphere for its equilibrium condition as static atmosphere. Indeed, Loschmidt made his calculation on the basis that gravitational heating would cause warm air at lower altitudes to rise and that in doing so he should follow a given number of moles of gas as it rose. As a consequence, the volume expansion of the gas as it rises causes it to cool on top of the static gravitational temperature gradient, so his prediction of the equilibrium temperature gradient is substantially too large for the static air condition. Indeed, the adiabatic pathway in a Carnot cycle for a perfect gas implies both a change of pressure and of volume for the gas. The temperature gradient calculated on the basis of energy conservation exists with still air and will be modified by dynamic conditions such as convection and wind due to energy gradients. The dynamic condition envisioned by Loschmidt occurs because of an energy gradient. The static air equilibrium temperature gradient occurs within an equal energy column of air. To calculate the static temperature gradient due to gravity, we must remember that temperature is an intensive, not an extensive parameter. Temperature is due to the energy of a molecule of gas, at least if it is a perfect and ideal gas as air nearly is. We are of course talking about a mean molecular energy in a given volume of air.
Of course in the real world, the static air equilibrium temperature gradient is a baseline and as we know air does rise by convection in variable amounts through a day. To the extent that air in our observed column has large amounts of air from the bottom rising and then expanding as it will often do under normal unstable conditions, an additional rate of cooling will occur. When all the air in the column is moving adiabatically, then the Loschmidt temperature gradient of about 9.78K/km will apply. For intermediate levels of air convection, the temperature gradient will vary from 6.49K/km to 9.78K/km. We also know that when the moisture content of air is high, it is lighter and upward convection tends to increase due to even less perturbation. The convection of moist air will affect the temperature gradient.
Heat Transport Mechanisms in the Lower Troposphere
Note that
this large surface warming by the action of the gravitational field depends
upon the surface not being in radiative equilibrium with the upper
atmosphere. It is the high density of
our atmosphere that produces this condition, in conjunction with the fact that
the mean free path for infrared absorption at the wavelengths that water vapor
and carbon dioxide absorb is very short due to their already substantial
concentrations. The absorption mean free
path for carbon dioxide is variously reported as 25, 33, and 47 m. That for water vapor is more variable, but on
average it is much shorter with an average value near 8 m. This has the
effect that the IR surface emission energy at wavelengths water vapor and
carbon dioxide can absorb is subject to dissipation amongst the more common
nitrogen and oxygen molecules where the collision frequency is high enough that
re-emission as IR radiation takes several times longer than the mean time between gas
molecule collisions. This rapidly brings
the greenhouse gases into equilibrium with the local air temperature, which
falls with altitude.
The fact that water evaporation and transport and air conduction, convection, and wind keep the surface from being in radiative equilibrium with the upper atmosphere is essential. Yet, there must also be infrared-emitting molecules in the upper atmosphere in sufficient quantity to establish a radiative equilibrium with space and our primary heating source, the Sun above a zone in which slower heat transfer mechanisms dominate. On Earth, this condition is established by our plentiful nitrogen, oxygen, and argon filled atmosphere and the presence of the dominant water vapor infrared emitter. The altitude in radiative equilibrium with space is primarily dependent upon the density of the lower atmosphere non-radiating gases and the rate of density change with altitude and the upper range of the dominant IR-active gas, water vapor. The doubling of a minor IR-absorbing and emitting gas such as carbon dioxide has little effect upon the altitude of the sphere in effective radiative equilibrium with space, especially when it emits from much higher altitudes and on the border with the tropopause.
The fact that water evaporation and transport and air conduction, convection, and wind keep the surface from being in radiative equilibrium with the upper atmosphere is essential. Yet, there must also be infrared-emitting molecules in the upper atmosphere in sufficient quantity to establish a radiative equilibrium with space and our primary heating source, the Sun above a zone in which slower heat transfer mechanisms dominate. On Earth, this condition is established by our plentiful nitrogen, oxygen, and argon filled atmosphere and the presence of the dominant water vapor infrared emitter. The altitude in radiative equilibrium with space is primarily dependent upon the density of the lower atmosphere non-radiating gases and the rate of density change with altitude and the upper range of the dominant IR-active gas, water vapor. The doubling of a minor IR-absorbing and emitting gas such as carbon dioxide has little effect upon the altitude of the sphere in effective radiative equilibrium with space, especially when it emits from much higher altitudes and on the border with the tropopause.
Of course there is no sharp shell at 5100 m which absorbs all solar insolation
and emits all the outgoing infrared radiation as from a simple black body
radiator shell. But, in trying to cut
through the many complexities of the Earth’s temperature balance, such a
picture makes very good sense for the purpose of understanding and estimating
the first-order effects on the Earth’s surface temperature. It offers a simple model which allows the
primary means of transferring energy by water evaporation, gas molecule
collisions, and other non-radiative effects to operate where they are the major
factors and leaves IR radiation the primary factor above about 4000 meters
altitude. Of course, there are frequency
windows in which most of the IR radiation emitted at the Earth’s surface can
escape straight into space.
IR radiative
cooling of the Earth by so-called greenhouse gases is strongest from about 4000
to about 11000 meters altitude and with the temperature dropping throughout the
troposphere with increasing altitude, radiative cooling becomes less and less
efficient. But it is rapid compared to the cooling effects of the lower troposphere. Data from the NIMBUS
satellites of the Earth’s emission spectrum into space show that the dominant water
vapor emission is mostly from altitudes from 2.5 km to 6 km, CO2
emission is from 3.5 km to 20 or more km with most of it in the beyond 10 km
altitude, and methane and nitrous oxide radiate mostly from 2 to 4.5 km
altitude. The methane and nitrous oxide
tend not to build up, since they are quickly broken down by UV radiation. Note that because CO2 reabsorbs its
emissions at lower altitudes or often has those emissions reabsorbed by water
vapor, it is only from the upper edge of the troposphere that CO2
emissions manage to reach space. The temperature at the top of the troposphere
has fallen to a frigid 217K. As a
result, the altitude with the temperature matching the thermal equilibrium seen
from space of 255 K is found at the top of the water emission zone at about
5000 m.
Having taken
into account the fact that the surface emissivity is close to 0.5, rather than
the black body value of 1.0, the temperature gradient in the troposphere due to
gravity, and understanding that radiative cooling of the Earth as a whole
occurs mostly from the top of the high concentration water vapor portion of the
atmosphere, we need to examine more issues relating to adding more of an
IR-active gas to the atmosphere and to more issues relating to backscatter
radiation warming of the surface. We
also need to appreciate the ability of non-radiative cooling mechanisms to keep
mid-day temperatures at the surface from soaring. It is clear that the major actors in
explaining this mid-day cooling are the evaporation of water at the surface,
conduction of heat across a thin layer of air very near the surface, and air
convection and winds toward the cooler polar regions. As noted, there is infrared surface emission
cooling also.
The Absorption of Solar Insolation in the Atmosphere
The Absorption of Solar Insolation in the Atmosphere
We need to
discuss the effects of the atmosphere upon incoming solar radiation. Rayleigh scattering by the atmosphere shields
the surface of a substantial portion of the UV and short wavelength visible
portion of solar radiation. Ozone
shields the surface from further UV radiation.
Water vapor and oxygen have absorption bands in the long wavelength
visible portion of the solar spectrum.
When
discussing any effects of IR-absorbing gases, one needs to take into account
the absorption of IR radiation incident on the Earth's atmosphere from the sun,
which is commonly very cavalierly not considered in comparison to the
back-reflection argument by strong greenhouse gas effect advocates. This is important, since much of the sun's IR
radiation does reach the Earth's surface and does warm it directly, though some
is also absorbed in the atmosphere before reaching the surface. In addition, some of the sun's IR radiation
is reflected by the surface, instead of being absorbed, so it does not directly
warm the surface. So, the question
arises: Do these IR-absorbing gases in
the atmosphere result in a net warming or cooling of the Earth's surface? If the absorption of solar insolation is
minimal and the back-radiation is as large as the man-made global warming
advocates have often claimed it to be, it might have a warming effect. Of course we now know that if it has a
warming effect, then there must be more cooling by air conduction and
convection and by water evaporation than they claim there is, since we now know
that direct solar insolation absorbed and the equilibrium temperature gradient
due to gravity is sufficient to explain why the surface temperature is about
15ºC.
First of all,
let us enlarge the context of the discussion.
The primary source of heat for the surface of the Earth is the radiant
energy of the sun. The solar wind of the
sun, materials dumped into the atmosphere from space, heat from the deep
interior of the earth, the interplay of changes in the Earth's magnetic field
and the sun's magnetic field, frictional warming due to winds across the
surface, the energy from the tidal effects of the gravitational interaction
with the moon and the sun are also contributors of energy or heat, though the
sum of these is very small compared to the sun's radiant energy spectrum of
ultraviolet (UV), visible, and infrared (IR) light. Nonetheless, in conjunction with the very
important variations in cloud cover and the less important effects of blown dust
and volcanic emissions these natural effects cause some of the variability in
the energy supply that affects the Earth’s surface temperatures.
The common
explanations for a catastrophic greenhouse gas hypothesis claim the effects of
the greenhouse gases upon the much more energetic incident UV, visible, and IR
portions of this spectrum of radiation from the sun are negligible. It is hard to comprehend how this critical
effect is given little attention and is so underestimated. A contributing reason is probably the fact
that a small percentage absorption from the solar insolation spectrum is likely
to occur in a much higher energy portion of the radiative spectrum compared to
the Earth’s surface emission spectrum.
Therefore the equivalent power percentage of the much lower power
spectrum of the Earth’s emissions would be large.
UV light is
11% of the radiant energy from the sun, if the UV range is that below 400 nm.
The UV light variance of 0.5 to 0.8% with the solar cycle is much larger
than is the visible light variance of 0.22%.
UV light is absorbed throughout the atmosphere, but much still reaches
the ground and is absorbed there. The
amount of UV radiation absorbed in the upper atmosphere is dependent upon the
amount of ozone there. The amount of
ozone is said variously to be dependent upon the solar wind, CFCs, water vapor,
and volcanic activity. When UV light is
more absorbed in the stratosphere than the ground, its surface warming effect
is greatly diminished. Much of the
absorbed energy is re-emitted as UV radiation and half of that energy is
quickly lost to space. Nonetheless, much
of the UV light energy is absorbed by the ground. In addition to the absorption of UV by ozone, it is also absorbed and re-emitted by electronic transitions by nitrogen, oxygen, argon, and carbon atoms.
It is often
incorrectly said that the entire atmosphere is transparent to visible light
which is the form of 40% of the radiant energy from the sun, taking visible light from 400 to 750 nm. Most people can actually see visible light from 370 to 770 nm and I can see it from at least 354 to 794 nm. Because of this, the visible light range is taken differently in different accounts.
Visible light is reflected from clouds and aerosol particles, but as we will see below, a considerable fraction of the visible light does not reach the ground or oceans to warm their surfaces even when the sky is clear. O2, atomic oxygen, and O3 absorb solar UV light. O3, O2, and H2O absorb some visible light from the solar insolation. The main O2 absorption is just about at the boundary between visible and infrared radiation, though I can personally see that wavelength. Water vapor and carbon dioxide are the main absorbers of solar insolation in the near (shortwave) infrared solar spectrum. The UV radiation is of higher energy than the visible light and the visible light is of higher energy than the near infrared radiation. The excitation of electronic transitions occurs in argon, carbon, oxygen, nitrogen atoms in the visible light range, so one has to consider these absorptions in addition to the vibrational molecular absorptions considered for water vapor.
We can see the absorption effects of the main atmospheric gases below, where shorter wavelength is higher energy. The UV portion of the spectrum is from 0.1 to 0.4 µm wavelength, the visible portion is from 0.4 to 0.75 µm wavelength, and the near infrared portion of the spectrum is from 0.75 to 3 µm wavelength. This covers the portion of the energy spectrum in which the solar insolation energies are important. Radiation from the Earth’s surface due to its temperature has a spectrum that peaks in the mid-infrared spectrum and has a significant tail into the far-infrared (longwave) spectrum. This emission spectrum is in a much lower energy range than is the solar insolation spectrum.
Visible light is reflected from clouds and aerosol particles, but as we will see below, a considerable fraction of the visible light does not reach the ground or oceans to warm their surfaces even when the sky is clear. O2, atomic oxygen, and O3 absorb solar UV light. O3, O2, and H2O absorb some visible light from the solar insolation. The main O2 absorption is just about at the boundary between visible and infrared radiation, though I can personally see that wavelength. Water vapor and carbon dioxide are the main absorbers of solar insolation in the near (shortwave) infrared solar spectrum. The UV radiation is of higher energy than the visible light and the visible light is of higher energy than the near infrared radiation. The excitation of electronic transitions occurs in argon, carbon, oxygen, nitrogen atoms in the visible light range, so one has to consider these absorptions in addition to the vibrational molecular absorptions considered for water vapor.
We can see the absorption effects of the main atmospheric gases below, where shorter wavelength is higher energy. The UV portion of the spectrum is from 0.1 to 0.4 µm wavelength, the visible portion is from 0.4 to 0.75 µm wavelength, and the near infrared portion of the spectrum is from 0.75 to 3 µm wavelength. This covers the portion of the energy spectrum in which the solar insolation energies are important. Radiation from the Earth’s surface due to its temperature has a spectrum that peaks in the mid-infrared spectrum and has a significant tail into the far-infrared (longwave) spectrum. This emission spectrum is in a much lower energy range than is the solar insolation spectrum.
It is not generally understood among those who discuss man-made global warming that electronic transitions do occur in the near infra-red range from 750 nm to 3000 nm. The emission and absorption spectra for neutral nitrogen, oxygen, argon, and carbon atoms are rich in spectral lines. These energies of these electronic transitions throughout the ultraviolet, visible, and near infra-red radiation range are listed in a major section of the Handbook of Chemistry and Physics. My 71st Edition lists the following numbers of emission lines for neutral atoms in the near infra-red spectral range:
Nitrogen, 55 emission lines from 739.864 to 1,787.826 nm
Oxygen, 87 emission lines from 770.675 to 2,617.356 nm
Argon, 84 emission lines from 750.3869 to 2,396.652 nm
Carbon, 39 emission lines from 786.089 to 1,972.199 nm
The strongest of the emission lines are those of argon, which is about 23 times more prevalent in the atmosphere than is carbon dioxide. The strength of the absorptions per atom appear to be in this order: argon, carbon, oxygen, and then nitrogen. The relative effects of argon and carbon dioxide have to take into account the 3 atoms per molecule of carbon dioxide, but that effect for argon is still considerably greater than for carbon dioxide. Generally the stronger absorption and emission intensities are found for the higher energy or lower wavelengths even of the near-IR range for these electronic transitions. Changes in the composition of the atmosphere must take into account the added absorption effects that each of these atoms has on incoming solar radiation by virtue of both electronic transitions and the excitation of vibrational modes in molecules. The added absorption of incoming solar radiation due to increasing carbon dioxide through electronic excitations is a cooling effect upon the surface temperature.
Finally, mid-IR radiation (3,000 to 30,000 nm) is not absorbed by nitrogen, oxygen, and argon gases which make up 99% of the atmosphere. Despite the electronic excitations of all of the atmospheric atoms, a large fraction of the solar IR directly warms the Earth's surface. Substantial amounts are absorbed by the dominant IR-absorbing gas, water vapor, and small amounts are absorbed by the very low concentration gas carbon dioxide. Methane and nitrous oxide mostly absorb the lower energy, longer wavelength infrared emissions from the Earth’s surface.
The incoming IR radiation absorbed in the atmosphere is much less effective in warming the Earth's surface than is that which is absorbed by the Earth's surface directly. This is because much of the absorbed energy locally warms a mass of air and it then rises as it expands and becomes more buoyant. Some of this energy absorbed in the atmosphere then is radiated again as IR radiation, but now half of that is directed out to space. That directed downward is quickly absorbed by the dense atmosphere and converted into rising convection. In other words, more water vapor and CO2 in the atmosphere results in a less effective warming of the surface because incoming solar energy is kept far from the surface. The principal IR-absorbing gases of water vapor and carbon dioxide have a cooling effect on the ground on the original solar radiance spectrum for portions of the 49% of the solar energy in the IR frequency range. This energy is still being deposited in the Earth's atmosphere, but has a much reduced effect in warming the Earth's surface.
Nitrogen, 55 emission lines from 739.864 to 1,787.826 nm
Oxygen, 87 emission lines from 770.675 to 2,617.356 nm
Argon, 84 emission lines from 750.3869 to 2,396.652 nm
Carbon, 39 emission lines from 786.089 to 1,972.199 nm
The strongest of the emission lines are those of argon, which is about 23 times more prevalent in the atmosphere than is carbon dioxide. The strength of the absorptions per atom appear to be in this order: argon, carbon, oxygen, and then nitrogen. The relative effects of argon and carbon dioxide have to take into account the 3 atoms per molecule of carbon dioxide, but that effect for argon is still considerably greater than for carbon dioxide. Generally the stronger absorption and emission intensities are found for the higher energy or lower wavelengths even of the near-IR range for these electronic transitions. Changes in the composition of the atmosphere must take into account the added absorption effects that each of these atoms has on incoming solar radiation by virtue of both electronic transitions and the excitation of vibrational modes in molecules. The added absorption of incoming solar radiation due to increasing carbon dioxide through electronic excitations is a cooling effect upon the surface temperature.
Finally, mid-IR radiation (3,000 to 30,000 nm) is not absorbed by nitrogen, oxygen, and argon gases which make up 99% of the atmosphere. Despite the electronic excitations of all of the atmospheric atoms, a large fraction of the solar IR directly warms the Earth's surface. Substantial amounts are absorbed by the dominant IR-absorbing gas, water vapor, and small amounts are absorbed by the very low concentration gas carbon dioxide. Methane and nitrous oxide mostly absorb the lower energy, longer wavelength infrared emissions from the Earth’s surface.
The incoming IR radiation absorbed in the atmosphere is much less effective in warming the Earth's surface than is that which is absorbed by the Earth's surface directly. This is because much of the absorbed energy locally warms a mass of air and it then rises as it expands and becomes more buoyant. Some of this energy absorbed in the atmosphere then is radiated again as IR radiation, but now half of that is directed out to space. That directed downward is quickly absorbed by the dense atmosphere and converted into rising convection. In other words, more water vapor and CO2 in the atmosphere results in a less effective warming of the surface because incoming solar energy is kept far from the surface. The principal IR-absorbing gases of water vapor and carbon dioxide have a cooling effect on the ground on the original solar radiance spectrum for portions of the 49% of the solar energy in the IR frequency range. This energy is still being deposited in the Earth's atmosphere, but has a much reduced effect in warming the Earth's surface.
A mid-day
solar light spectrum outside the atmosphere and the solar radiance spectrum
transmitted through the atmosphere to sea level in the South Pacific are shown
in Fig. 4. The outside the atmosphere
solar spectrum is not quite that of a black body at the near surface temperature of
the sun, because some absorption in radiation from the sun occurs in its cooler
surface plumes, the solar wind, and by
the extremely low concentration gases of the solar system due to the large
distance from the sun to the Earth.
The
measurement of the transmitted energy from space to the Earth’s surface and its
distribution with wavelength is highly dependent upon the amount of water vapor
in the atmosphere, so the transmitted spectrum may vary considerably, but the
spectrum shown is fairly typical. But,
for the purposes of this discussion, let us use the overall transmittance
values to the Earth's surface from this graph of an actual particular
measurement. This is not an average, but
it makes the point that such real effects must be accounted for and have a
major impact on the argument of whether IR-absorbing gases heat or cool the
surface of the Earth. The overall energy
transmittance is about 0.65, which is in good agreement with the accepted
average. The transmittance of UV and
Visible radiation combined is about 0.59, while that for IR radiation is about
0.69 according to the limited range of the graph in Fig. 4. The total fraction of the solar insolation
incident at the top of the Earth’s atmosphere and transmitted by the atmosphere
and incident upon the surface here is then (0.59)(0.51) + (0.69)(0.49) = 0.64,
which shows the breakdown by portions of the spectrum to be within round-off
error of the overall transmittance. This
is slightly higher than the 0.58 fraction of the Kiehl-Trenberth diagram in
Fig. 2, but in good agreement with many other sources.
The 31% loss
of solar insolation IR radiation in the atmosphere measurable from Fig. 4 is
due to water vapor and CO2. However,
absorption in the long IR tail beyond the 2700 nm cut-off on Fig. 4, shows much
higher IR absorption in that tail. These
IR-absorbing gases are keeping at least 31% of the 49% of the solar radiation
due to IR from reaching the Earth’s surface.
Thus, at least (0.31)(0.49) = 0.152 of the total solar IR radiation
incident upon the top of the atmosphere does not reach the ground because of IR
absorption. But due to the neglect of
the long IR tail with its higher level of absorption due to water vapor and CO2,
a better value for the fraction of the solar insolation IR absorbed by the
atmosphere is 19%, which is pointed out in the very useful paper by Klaus
Ermecke entitled Rescue from the Climate Saviors, published in June 2010 by KE
Research.
This provides
a sizeable cooling effect upon surface temperatures attributable to the
so-called greenhouse gases of water vapor and carbon dioxide. If they did not absorb this solar insolation,
the additional power incident upon the surface would be (0.19)(342 W/m2)
= 65.0 W/m2. Add this to the 219
W/m2 (64% of 342 W/m2) actually incident upon the surface
and assume that the surface reflectivity is still 15.2% as used by K-T in Fig.
2., then the total power absorbed by the surface would be (1 - 0.152) (219 +
65) W/m2 = 241 W/m2.
With a surface emissivity of 0.5, this would make the surface temperature
303.6K. This means that the absorption of incoming solar radiation by water
vapor and carbon dioxide is a 16.0K cooling of the surface. This is substantially
more than the IPCC claim for the temperature rise due to doubling the CO2
concentration in the atmosphere of 5.4K with strong positive water vapor reinforcement.
This brings home the critical need to account for additional cooling
absorption of the IR portion of solar insolation due to changes in the water
vapor and carbon dioxide concentrations in the atmosphere.
Further
increases in the CO2 concentration will add to this cooling effect
by preventing still more solar insolation in the IR range from reaching the
surface. What is more, the claimed 5.4K
temperature increase due to doubling the CO2 concentration depends
upon the correctness of the IPCC claim that there is a strong positive feedback
causing water vapor increases. Water vapor increases are said to cause all but 1.2K
of that 5.4K temperature increase.
If it were true that water vapor did increase due to increased CO2, then water vapor would definitely block more surface absorption of solar insolation as an IR-absorber and it would generate more cloud cover, which would reflect more solar insolation from well up in the atmosphere off into space. Cloud cover is a powerful coolant for the surface temperatures. These effects of added water vapor make it most unlikely that water vapor has a strong positive feedback effect upon increased carbon dioxide supposed warming. That additional water vapor is a powerful coolant in the lower atmosphere is also well known from the fact that the humid air lapse rate, the measured temperature gradient with altitude, is lower than the dry air lapse rate. Indeed, the added IR absorption of solar insolation caused by CO2 itself would reduce the amount of warming CO2 might produce by some other mechanism.
If it were true that water vapor did increase due to increased CO2, then water vapor would definitely block more surface absorption of solar insolation as an IR-absorber and it would generate more cloud cover, which would reflect more solar insolation from well up in the atmosphere off into space. Cloud cover is a powerful coolant for the surface temperatures. These effects of added water vapor make it most unlikely that water vapor has a strong positive feedback effect upon increased carbon dioxide supposed warming. That additional water vapor is a powerful coolant in the lower atmosphere is also well known from the fact that the humid air lapse rate, the measured temperature gradient with altitude, is lower than the dry air lapse rate. Indeed, the added IR absorption of solar insolation caused by CO2 itself would reduce the amount of warming CO2 might produce by some other mechanism.
Fig. 5. The percentage of blue sky observed by
satellite between 1983 and 2009 from the paper by Klaus Ermecke, Rescue from
the Climate Saviors, June 2010, KE Research.
Note that the blue sky percentage was low in the cooler 1980s, was high
in the warming period after 1996, but has been falling slightly since about
2002 as the warming has paused. The
range of variation is from about 30.5% to 36%, with the high about 18% greater
than the low. Bigger variations have
likely occurred in the past. At least in
this time frame, increased cloud cover correlates with cooler surface
temperatures, which is hardly surprising.
Increased water vapor in the atmosphere will generally form more clouds
as rising warm air cools and the water vapor condenses.Thus, increased water vapor owing to warming effects already underway produces more cloud cover, which cools the Earth's surface. The general effect of increased water vapor is both to absorb and to reflect more of the solar insolation before it reaches the surface. This cools the surface and demonstrates the usual negative feedback of water vapor to warming caused by other factors such as increased solar insolation at the top of the atmosphere or any possible increase due to increased carbon dioxide.
In each case,
whether UV, visible light, or IR, not all of the radiation of that form
striking the Earth's surface is absorbed.
Some fraction is reflected and the fraction is very dependent on whether
the ground is covered with snow, plowed earth, grasses, forests, crops, black
top, or water. There are real ways that
man does have some effect on the Earth's temperature. He changes the surface of the earth over a
fraction of the 30% of its surface which is land, affecting its reflectivity,
its contributions to evaporative cooling, thermal convection, and its local
emissivity. He also converts fossil and
biomass fuels into heat. The carbon black
and other small particles he releases into the atmosphere and some aerosols man
generates, also have some impact on the temperature at the Earth's
surface. His use of the land may affect
the amount of dust which is blown, sometimes for long distances. Compared to the overall natural effects,
these man-made effects are small, yet they may be larger than the effect of man
adding CO2 and methane to the atmosphere for reasons we have and are
about to further develop.
In the outer,
low density atmosphere, the primary means of heat transfer is radiant transfer
by IR emission from an energetic molecule or atom, since collisions of
molecules and atoms for direct energy transfer have mean times between events
greater than the time between an IR-absorbing gas absorbing and then
re-emitting IR radiation. In the denser,
lower altitude atmosphere, most energy transfer is due to gas molecule
collisions and the convective flow of masses of warmed air. Near the Earth's surface, much of the energy
lost by the warmed surface is due to gas molecules striking the surface and
picking up heat and then colliding with other molecules to transfer heat from
one to another or due to the evaporation of water. Radiative cooling of the surface is
important, but due to IR-absorbing gas molecules such as water vapor and carbon
dioxide, most of that energy is reabsorbed by the atmosphere only a few tens of
meters from the surface. This means
there is a bit of a speed up in the removal of heat in that IR over those few
tens of meters travels at the speed of light, not at the speed of air
convection currents or wind. That
portion of the surface emission spectrum not absorbed by IR-absorbing gases is
simply emitted off into space at the speed of light. Thus, radiative cooling is extremely
efficient without greenhouse gases, but still efficient with them when compared
to air convection. This radiative
efficiency in cooling is very apparent in dry deserts and at high elevations on
a mountain in the night and is easily experienced.
Once a body
of air near the surface is heated, then masses of warmed air molecules are
transported upward into the cooler atmosphere at higher altitudes or laterally
toward cooler surface areas by convection or wind. Warmed molecules, most of which are nitrogen,
oxygen, and argon, will act to keep the rarer water vapor and carbon dioxide
molecules at the same temperature they are at for a particular altitude. These water vapor and other IR-absorbing
molecules will emit IR radiation in the mid and far infrared ranges. However, no molecule or atom at a low
temperature such as that near the Earth's surface is a very effective energy
radiator, since the Stephan-Boltzmann equation depends upon the fourth power of
the absolute temperature, which commonly near the Earth's surface is about 288K. As molecules rise in altitude, their
temperature falls and they become still less efficient IR emitters. The number of such emitters falls as the
atmospheric density falls, but the mean free path before re-absorption of an
emitted photon occurs becomes longer. Thus,
gas molecule collisions and convection and the evaporation of water and its
transport are the dominant means of heat transfer in the dense atmosphere near
the surface. These processes on balance
cool the surface of the Earth and redistribute some of the heat back into the
upper troposphere and cooler places such as those shaded from the sun or in
arctic regions.
Substantial Surface Radiation Power Conversion to Other Cooling Mechanisms
Substantial Surface Radiation Power Conversion to Other Cooling Mechanisms
You might be
thinking there is a contradiction in the above paragraph in which I say that
water evaporation and air convection are the primary means of energy transport,
but earlier I said that the radiation from the surface was as great as 196 W/m2. First of all, because this is not surface
emission into a vacuum, the radiation potential given by the Stefan-Boltzmann
equation is not realized. Other cooling
mechanisms remove much of the energy that would otherwise be radiated into a
vacuum. Even that portion which is
radiated as IR is soon converted into other forms of energy transport. This is because the absorption length for
that part of the emitted surface radiation which can be absorbed by greenhouse
gases in the lower atmosphere is very short.
Absorbed energy is soon spread to the far more plentiful non-IR-absorbing
gases.
The
partitioning of the energy between radiation and both conduction and convection
changes rapidly even in the first 50 m above the surface. This is because the mean free path for IR
radiation absorbed by water vapor at sea level is only 8 m and that for carbon
dioxide is 47 m according to the calculations of Nasif Nahle in his July 2010
paper entitled Mean Free Path of Photons through the Troposphere and Time of
Crossing Path of Photons Leaving the Troposphere Without Colliding with a Molecule
of Carbon Dioxide and/or a Molecule of Water Vapor.
In one mean free path distance, the number of unabsorbed photons is about 0.368 times the initial number. At a low altitude of only 100 meters, the fraction of unabsorbed photons emitted from the ground at a water absorbing frequency by water vapor averages about 3.7 x 10-6. Those IR photons emitted at frequencies absorbed by carbon dioxide would be reduced to 12% of their initial number by absorption by CO2, if CO2 did not often absorb at frequencies also absorbed by water vapor. With more water vapor, the loss of photons that can be absorbed by CO2 will occur even more rapidly.
This is why, when coupled with a high molecular collision rate of 6.92 billion collisions a second at sea level, surface radiative energy is very rapidly converted into much slower moving energy transport by air conduction and convection. Consequently, the height above the surface at which the measurements of surface radiation versus air conduction and air convection are made will result in large variations in the partition of energy transport between these mechanisms. A measurement made 1 meter above the surface will differ greatly from one made 10 meters or 50 meters or 100 meters or 200 meters above the surface in the ratio of surface radiation to air conduction and convection. It will also depend strongly upon the humidity of the air.
In one mean free path distance, the number of unabsorbed photons is about 0.368 times the initial number. At a low altitude of only 100 meters, the fraction of unabsorbed photons emitted from the ground at a water absorbing frequency by water vapor averages about 3.7 x 10-6. Those IR photons emitted at frequencies absorbed by carbon dioxide would be reduced to 12% of their initial number by absorption by CO2, if CO2 did not often absorb at frequencies also absorbed by water vapor. With more water vapor, the loss of photons that can be absorbed by CO2 will occur even more rapidly.
This is why, when coupled with a high molecular collision rate of 6.92 billion collisions a second at sea level, surface radiative energy is very rapidly converted into much slower moving energy transport by air conduction and convection. Consequently, the height above the surface at which the measurements of surface radiation versus air conduction and air convection are made will result in large variations in the partition of energy transport between these mechanisms. A measurement made 1 meter above the surface will differ greatly from one made 10 meters or 50 meters or 100 meters or 200 meters above the surface in the ratio of surface radiation to air conduction and convection. It will also depend strongly upon the humidity of the air.
The solar
irradiance has a power density just outside the atmosphere of the Earth of
about 1367 W/m2. We saw from
the discussion of the transmittance spectrum of the sun's radiation that the
overall energy reaching the surface is about 0.65 times the total energy outside
the outer atmosphere. So 0.65 times 1367
W/m2 is 888.6 W/m2, which reaches the Earth's
surface. Of this energy, about 15.2 % is
reflected from the Earth's surface without being absorbed according to the
Kiehl-Trenberth energy balance diagram above.
Thus, the
energy warming the surface is the absorbed power density of about 753.5 W/m2 at the time of
maximal solar insolation during an average day.
With a surface emissivity of the Earth of 0.5, the temperature of the
surface would be 404K or 131ºC, were it not for conduction of heat into the
subsurface, evaporation of water, and air conduction and convection cooling of
the surface! Such a temperature would be
fatal for humans and most of the Earth’s surface life-forms. Humans would be boiled to death. This tells us how critically important it is
that these surface cooling mechanisms are very powerful when they need be. Since the daily high is rarely higher than
about 106ºF or 314 K, these cooling mechanisms can generally lower the surface
temperature by more than 90K.
If the surface radiation were the strong surface cooling effect shown in the Kiehl-Trenberth Energy Budget to altitudes of thousands of meters and back radiation from the atmosphere existed at the hugely exaggerated power densities shown in that diagram, some very interesting and terrible things would happen at mid-day. Just taking the absorbed power ratio to the surface emitted power ratios would give (753.5 W/m2) / (168 W/m2) = P / (390 W/m2), so P = 1749 W/m2.
Assuming human skin absorbs all such IR radiation as the K-T model claims the Earth's surface does, then such mid-day surface radiation would surely cook our goose! Since we are largely water based organisms as are plants covering most of the land surface, we ought to have similar absorption properties to those they claim the land portions of the Earth have. Standing in bright mid-day sun we have all felt the substantial warming of the 753.5 W/m2 from the direct line with the sun, but we do not feel the even greater 1749 W/m2 coming up from the ground we should expect under the K-T physics.
Dissipation of Surface IR Emission Heat by IR-Active Gases and Collisions
If the surface radiation were the strong surface cooling effect shown in the Kiehl-Trenberth Energy Budget to altitudes of thousands of meters and back radiation from the atmosphere existed at the hugely exaggerated power densities shown in that diagram, some very interesting and terrible things would happen at mid-day. Just taking the absorbed power ratio to the surface emitted power ratios would give (753.5 W/m2) / (168 W/m2) = P / (390 W/m2), so P = 1749 W/m2.
Assuming human skin absorbs all such IR radiation as the K-T model claims the Earth's surface does, then such mid-day surface radiation would surely cook our goose! Since we are largely water based organisms as are plants covering most of the land surface, we ought to have similar absorption properties to those they claim the land portions of the Earth have. Standing in bright mid-day sun we have all felt the substantial warming of the 753.5 W/m2 from the direct line with the sun, but we do not feel the even greater 1749 W/m2 coming up from the ground we should expect under the K-T physics.
Dissipation of Surface IR Emission Heat by IR-Active Gases and Collisions
Let us next
examine the portions of the Earth’s radiation spectrum which are absorbed and
re-emitted by IR-active gases so we can better assess claims of a large
back-radiation effect, despite the fact that such an effect would force us to
posit compensating non-radiative cooling effects. See Fig. 6. below.
Fig. 6. The red spectrum of UV, visible light, and
near infrared radiation is that from the sun and incident upon the Earth, while
the blue spectrum at the top of the diagram is the mid and far infrared
radiative spectrum of the Earth. The
outer curves are those of black body radiators at the stated temperature, while
the interior solid red spectrum is the radiation incident upon the Earth’s
surface and the solid blue spectrum is the radiation from the surface which is
not absorbed by the atmosphere. It is critical to note that there are two deceptions in this figure. One is that the area under the solar energy spectrum incident upon the surface is about four times greater than the area under the Earth surface emission spectrum. The other deception to the eye is the fact that the wavelength is given on a logarithm scale. This compresses the wavelength on the right side of the scale. Because the energy of a photon is proportional to the inverse of the wavelength, this means the energy scale is stretched out on the right side. This makes it appear that IR-active gas absorption is much more important an effect on the emission side than on the solar insolation side. The figure
clearly shows that the Earth’s surface is not in radiative equilibrium with
space due primarily to absorption by water vapor and very secondarily due to
absorption by carbon dioxide. Note that
the solid blue radiation spectrum is not all the energy which is emitted into space, but only the part that was emitted from the surface into the atmospheric window. The radiation absorption spectra due
to various absorption mechanisms are shown in the lower portion of the diagram,
with their absorption sum shown above.
The absorption effects of water vapor and carbon dioxide are both fully
saturated over the majority of the Earth’s emission spectrum.
Note that the
solar radiation absorption spectrum at the top left shows somewhat less
absorption than the actual measurement in Fig. 4. This probably reveals that there is some
shortcoming in the approach of trying to reconstruct that absorption from the
separate absorption spectra of the gases considered here. Nitrogen gas, which is 78.084% of the
atmosphere, is entirely left out, because it is not an infrared absorber. Perhaps it has ionization products and dimer
or trimer products with water that do absorb, however. There is reason to believe that CO2
has such products. These and other
similar products of other gases may account for the additional absorption that
occurs in the measured solar radiance compared to the composed one of the
figure immediately above. But since this data is well-respected in catastrophic
greenhouse gas circles, it is fair to use it to at least show some of the
limitations of the usual explanations of the catastrophic greenhouse gas
hypothesis. The fraction of the long
wavelength IR emitted from the ground at about 290K which is absorbed as
actually shown in this figure is 0.65, though the labeling says it is from 0.70
to 0.85. We will take this fraction to
be the shown 0.65, consistent with the practice of many others.
A fraction of
the gas molecules which have absorbed long wavelength IR radiation emitted from
the ground will cool by emitting IR radiation in turn or by collisions with other molecules. Water vapor is the best long wavelength IR
absorber and it is the best emitter of IR energy, but before it can commonly
emit the energy it has absorbed from IR radiation, even it will likely suffer
numerous gas collisions with much of its excess molecular energy being
transferred in those collisions to the molecules which collide with the water
molecule. Nitrogen molecules are the
most likely molecules to take up much of the energy from the water molecule,
since nitrogen is 78.08% of the atmosphere.
Oxygen molecules are the next most likely colliders at 20.95% and then
argon atoms at 0.93%. Together, these
three gases account for 99.97% of the U.S. Standard Atmosphere. None of these gas molecules are IR absorbers
in the long wavelength, or mid and far infrared, spectrum.
At sea level,
the mean gas velocity is 459 m/s, the mean free path or distance between
collisions is only 6.6 x 10-8 m or 66 nm, and the collision
frequency is 6.9 billion/s. At an
altitude of about 4000 m, the radiative transfer of energy competes about
evenly with transfer by collisions. At 4000
m altitude, the frequency of gas molecule collisions is about 4.4
billion/s. This means the radiative re-emission process has an equivalent time of about 2.2 billion/s. We can use the equivalency of
energy transfer by radiation and gas molecule collisions at the 4000 meter
altitude to estimate the fraction of energy transfer by radiation of the total
of energy transferred by radiation plus gas molecule collisions.
At sea level, energy transfer by radiation is equivalent to about 2.2 x 109 collisions per second, so the fraction of energy transferred by radiation after the first absorption event by an IR-absorbing molecule is about 2.2/6.9 = 0.32 of the total by gas molecule collisions and radiation. This suggests that about 2 times as much energy is transferred by gas collisions as by radiation at sea level after one mean free path length for absorption.
At sea level, energy transfer by radiation is equivalent to about 2.2 x 109 collisions per second, so the fraction of energy transferred by radiation after the first absorption event by an IR-absorbing molecule is about 2.2/6.9 = 0.32 of the total by gas molecule collisions and radiation. This suggests that about 2 times as much energy is transferred by gas collisions as by radiation at sea level after one mean free path length for absorption.
Note that
this estimated contribution of sea level energy loss by radiation is much lower
than the huge losses in the K-T energy budget of Fig. 2. Their fraction of the energy loss of the
surface into the upper troposphere by radiation was 0.71. This is because surface radiation was given as 390
W/m2, evaporation was given as 78 W/m2, and convection
was given as 24 W/m2, but the absorbed surface radiation was 350 W/m2.
I place an upper limit on the surface radiation cooling of 196 W/m2 and would actually include the evaporation and the convection cooling in that number. As a consequence, if we assume the K-T estimates of evaporation and convection to be right for the sake of argument here, radiative cooling very, very near the surface is (196 – 78 – 24) W/m2 = 94 W/m2. This is only 0.48 of the total cooling. After a mean free path length for absorption of the surface emitted IR radiation at wavelengths that can be absorbed by water vapor or CO2, it is easy to see that the radiation fraction of such heat transport falls from 0.48 to 0.32.
I place an upper limit on the surface radiation cooling of 196 W/m2 and would actually include the evaporation and the convection cooling in that number. As a consequence, if we assume the K-T estimates of evaporation and convection to be right for the sake of argument here, radiative cooling very, very near the surface is (196 – 78 – 24) W/m2 = 94 W/m2. This is only 0.48 of the total cooling. After a mean free path length for absorption of the surface emitted IR radiation at wavelengths that can be absorbed by water vapor or CO2, it is easy to see that the radiation fraction of such heat transport falls from 0.48 to 0.32.
There is
another caveat of importance: the radiation transport is from emitters which
are essentially in thermal equilibrium with the gas molecules at its altitude
and therefore on a cooling gradient with increasing altitude. What is more, the radiation emitted is
absorbed by further molecules above which are only very slightly cooler because
they are not far above. When that
radiation is downward, the potential absorbers are usually warmer. When they are cooler, as
might be the case if that pocket of air is in the shade of a tree or
building, they may be warmed. Another case of downward
warming may be in the thin layer of air over a water surface that cooled during
the night and because of the high heat capacity of water, the air a few feet
over the water surface is warmed in the morning sun faster than is the surface of
the water.
Let us recall
that we earlier calculated that at most 196 W/m2 left the surface as
cooling IR radiation and that after subtracting the K-T evaporation and
convection amounts this is only 94 W/m2. Of the 94 W/m2 of surface leaving
IR, 65% is absorbed by greenhouse gases according to Fig. 6. Thus the gas-absorbed IR from the surface is at
most 61 W/m2 and only 32% of this is re-emitted as IR radiation by
the absorbing gas molecules due to the high collision rate. A few tens of meters from the surface, we now
know that no more than (0.32) (61 W/m2) = 19.5 W/m2 of IR
radiation is still being transported at those wavelengths that can be absorbed
by greenhouse gases. 35% of 94 W/m2,
or 33 W/m2, is IR radiation in the atmospheric window which cannot
be absorbed and is lost to space. 41.5 W/m2 of initial IR radiation
from the surface has been converted into convection transport or thermals due
to molecular collisions thanks to the so-called greenhouse gases. Then at most half of the 19.5 W/m2
of IR radiation that was absorbed by a greenhouse gas and re-emitted is sent
toward space and half is sent toward the ground.
So now we
have 9.25 W/m2 trying to work its way back to the surface, but it will
not get far before it is re-absorbed. This radiation was really less than 9.25
W/m2 anyway because it was emitted from ever cooler molecules the
higher up it had made its way before heading back toward the surface. Suppose that on average the back-radiation
was emitted by absorbing gas molecules nearly half way to the 5100 m altitude
effective radiative equilibrium altitude.
At 2500 m altitude the U.S. Standard Atmosphere temperature is 271.9K. Let us compare the radiative power ratio of
the gas at 271.9K to that at 288.15K. We
have:
P2500m
/ Psea level = (271.9K)4
/ (288.15K)4 = 0.793
Thus the
upper limit on the back-radiation is more like 0.793 (9.25 W/m2) = 7.3
W/m2. Some fraction of the
energy incident upon the ground is reflected, but even assuming this is
negligible as wrongly claimed by K-T, the upper limit on absorbed back radiation from that emitted from
the ground is 7.3 W/m2. Then
there is still the question of whether the surface can absorb this
back-radiation when it already is emitting radiation at the appropriate level
for a generally warmer body.
According to the
Kiehl-Trenberth diagram of Fig. 2, 67 W/m2 of solar insolation was
absorbed by the atmosphere. Some of this
absorbed energy is re-emitted as IR radiation toward the ground and some toward
space. Assuming it on average was
equilibrated with the surrounding air at 2500 m altitude and that half
is heading toward the surface, it must still traverse the last few tens of meters
of the dense atmosphere above the surface.
Only 32% of the absorbed IR in that layer of atmosphere over the
surface will be re-emitted as IR radiation and it will have
an effective power ratio not higher than 0.793.
Thus, an upper limit, assuming total absorption at the surface without
reflection, is (0.5) (0.32) (0.793) (67 W/m2) = 8.5 W/m2.
We now have
an upper limit for the IR radiation upon the surface which is not part of the
direct solar insolation. The upper limit
is (7.3 + 8.5) W/m2 = 15.8 W/m2. This is hugely less than the 324 W/m2
claimed in the Kiehl-Trenberth energy budget of Fig. 2.
The situation is actually far worse than this, because the radiative upper limit due to back radiation of the surface emitted and absorbable component was taken as a one step process with respect to the temperature gradient. If instead we as what the flux of radiation across a single mean free path length for water vapor of 8 m is, we find the radiative transfer of energy to be hugely reduced. As we discussed earlier, the temperature gradient is between 6.49 and 9.78K/km. Let us assume a case of the gradient being 9.78K/km. The temperature differential for a single 8 m radiation hop is then only 0.08K. If the surface is at a temperature of 287.65K and 8 meters above that the temperature is then 287.57K, the power flux is
The situation is actually far worse than this, because the radiative upper limit due to back radiation of the surface emitted and absorbable component was taken as a one step process with respect to the temperature gradient. If instead we as what the flux of radiation across a single mean free path length for water vapor of 8 m is, we find the radiative transfer of energy to be hugely reduced. As we discussed earlier, the temperature gradient is between 6.49 and 9.78K/km. Let us assume a case of the gradient being 9.78K/km. The temperature differential for a single 8 m radiation hop is then only 0.08K. If the surface is at a temperature of 287.65K and 8 meters above that the temperature is then 287.57K, the power flux is
P = 0.5 (5.6697 x 10-8 ) [(287.65)4 - (287.57)4 W/m2 = 0.22 W/m2 ,
and this is a flux in the wrong direction on average. Even with an inversion of the normal temperature gradient, it is very hard to imagine an absorbed back radiation of the scale of the 15.8 W/m2 upper limit calculated above.
To sum up the
situation of the power flux cooling the surface:
94 W/m2
leaves the surface as IR and may be lower if K-T numbers for evaporative and
convection cooling are too low
78 W/m2 leaves the
surface as evaporative cooling according to K-T
24 W/m2 leaves the
surface as convection (and conduction) according to K-T
15.8 W/m2 is the
maximum possible back-radiation warming of the surface and any part of it that
occurs necessitates an increase in one of the above cooling mechanisms
It is also
worth summarizing that the 94 W/m2 of IR radiative cooling power
from the surface takes the following forms just a few tens of meters above the
surface:
33 W/m2,
Outgoing IR radiation in the atmospheric window
9.25 W/m2,
upper limit of IR radiation in the wavelengths absorbed by greenhouse gases transporting heat upward and losing power
with altitude rapidly in the first couple of thousand meters. This energy flux may become less than 1 W/m2 before increasing again as the mfp increases and collision rates decrease.
51.75 W/m2,
Transported by air conduction, convection, and wind
I was concerned
that the 24 W/m2 of thermals at the surface according to Kiehl and
Trenberth made soaring hawks, eagles, and other birds absolutely supernatural
in their soaring ability, but it is clear that thermals increase with altitude
within a few tens of meters over the surface and soon become substantially
greater than the 24 W/m2 estimate by Kiehl and Trenberth. In fact, they appear likely to be about (24 + 51.75) W/m2 = 75.75 W/m2. Unmanned gliders have been developed that use a propeller to get off the ground and to get a couple of tens of meters in altitude. Once there, they can glide all day. The air is relatively still over the featureless ocean close to its surface, but an albatross can fly thousands of kilometers without flapping its wings because the layer of air just a bit higher is moving quite nicely. The albatross can swoop and climb all day using the wind shear between the surface layer of air and the air just above it. We humans are generally not aware of these effects and are prone to underestimating them.
Now some will
be reluctant to believe that the fraction of radiative cooling of the surface immediately at
the interface with the atmosphere is only about 48% of the total cooling,
assuming that back-radiation does no heating of the surface. Viewed from a short distance above the
surface, the radiation percentage has dropped to 42.25 / 196 = 0.22 or 22%. Yet, Physics Prof. Robert Williams Wood of
Johns Hopkins University in his classic greenhouse experiments concluded that
IR radiation from solar warmed surfaces was only about 4%. Chilingar, Khilyuk, and Sorohtin concluded in
2008 that surface radiation was only about 8% of the surface cooling. The value I have given here of 22% is an upper limit.
There is no
problem with the alternative cooling mechanisms being much larger than the low values given above. We
can get some insight on that by returning to the issue of the mid-day cooling
when as much as 753.5 W/m2 is being absorbed in the surface. On a hot day, the temperature at a
mid-latitude might be 106ºF or 41ºC or 314K.
The radiative cooling upper limit is then 275.6 W/m2 and is
likely only half of that at most. This
means that other cooling mechanisms, including the flow of heat into the ground
or underlying water, cool the surface with a power of about (753.5 – 0.5
(275.6)) W/m2 = 615.7 W/m2 at mid-day. At that time, the radiative cooling is not
more than 137.8 / 615.7 = 0.224 or about 22%.
Once again, this fraction of the radiative cooling may still be
influenced by a low average set of values for evaporative and convective cooling
by K-T. Consequently, it is easy to
believe that surface radiative cooling is less than 22% of surface cooling.
Let us examine Figure 6 to determine what the relative effects of CO2 absorption are on the solar insolation spectrum and on the Earth radiative emission spectrum. We must remember that Figure 6 is deceptive for this purpose because the amplitude of the solar insolation spectrum and the Earth emission spectrum have been normalized. It is also deceptive because the abscissa is not the energy scale we would desire for our purposes, but it is the logarithm of the wavelength. Because the energy of a photon is proportional to the inverse of the wavelength, this means the energy scale on the solar spectrum side is compressed, while the energy on the Earth emission side is expanded. When we look at the absorption of carbon dioxide below that of water vapor, the same distortions apply. Such plots are one of the reasons why so many scientists dismiss the importance of both water vapor and carbon dioxide absorption of incoming solar insolation and over-emphasize that of their absorption of the Earth's radiative emissions.
How do we adjust the amplitude of the solar insolation spectrum. Let us compare the solar insolation that passes into the atmosphere minus that reflected from the atmosphere to the surface emission. Using the K-T energy budget of Figure 2, the solar insolation into the atmosphere is (342 - 77) = 265 W/m2 and the surface emission should be (168 - 24 -78) W/m2 = 66 W/m2 . The ratio of the integrated areas under the curves, if they were plotted on an energy scale would then have to be about 265 / 66 = 4.02. For the moment, let us forget the problem of the abscissa not being linear in energy. We will just multiply the amplitude of the solar insolation curve by a factor of four.
Now let us examine the CO2 absorption plot by itself. Observe the four most intense peaks and note that if we have multiplied the amplitude of the solar insolation curve by four, then the third of the four largest CO2 absorption peaks from the left side has about the same effect on both the solar insolation spectrum and on the surface emission spectrum at about 287.65K. We will amplify the magnitude of the CO2 absorption peaks to its left by a factor of four. Comparing the four-fold increased area of those peaks in the energy range for the solar insolation with those in the surface emission energy range, one finds that the energy absorbed by CO2 from the solar insolation is about 1.3 times that absorbed from the surface emission spectrum. What is more, because the energy ranges on the solar insolation side are compressed and those on the surface emissions side are stretched, this is an under-estimate. CO2 by itself is clearly doing more to cool the surface by keeping solar energy from reaching it, than it absorbs on the emission side.
We also have to remember that even if the energy absorbed from the sun were equal to that absorbed from the surface, the effect would still really be a cooling of the surface. This is because of a built-in asymmetry in the energy transport processes. Contrary to the popular misconception, energy absorption by CO2 from the radiation in the surface emission spectrum does not warm the surface as we have discussed. This absorbed energy is doomed to follow the same path as the energy absorbed by the atmosphere out of the incoming solar insolation. That energy will percolate upward and be emitted from higher up in the atmosphere without affecting the surface temperature.
The Mean Atmospheric Radiative Altitude
There is
another reason to believe that radiative cooling from the surface is a small
fraction of the total cooling. This is
the very stability of the daily cycle temperature range we generally
experience. The surface cooling is
accomplished by slower energy transport mechanisms such as air conduction and
convection and water evaporation, the more moderate our daily temperature
excursions. If radiative cooling near
the surface really averaged 48% of all cooling, let alone the 71% of the K-T energy budget of Fig. 2., the
day to night temperature variations would surely be larger than they are.
Absorption Effect of Atmospheric CO2 on Solar Insolation Compared to Surface Radiative Emission
Absorption Effect of Atmospheric CO2 on Solar Insolation Compared to Surface Radiative Emission
Let us examine Figure 6 to determine what the relative effects of CO2 absorption are on the solar insolation spectrum and on the Earth radiative emission spectrum. We must remember that Figure 6 is deceptive for this purpose because the amplitude of the solar insolation spectrum and the Earth emission spectrum have been normalized. It is also deceptive because the abscissa is not the energy scale we would desire for our purposes, but it is the logarithm of the wavelength. Because the energy of a photon is proportional to the inverse of the wavelength, this means the energy scale on the solar spectrum side is compressed, while the energy on the Earth emission side is expanded. When we look at the absorption of carbon dioxide below that of water vapor, the same distortions apply. Such plots are one of the reasons why so many scientists dismiss the importance of both water vapor and carbon dioxide absorption of incoming solar insolation and over-emphasize that of their absorption of the Earth's radiative emissions.
How do we adjust the amplitude of the solar insolation spectrum. Let us compare the solar insolation that passes into the atmosphere minus that reflected from the atmosphere to the surface emission. Using the K-T energy budget of Figure 2, the solar insolation into the atmosphere is (342 - 77) = 265 W/m2 and the surface emission should be (168 - 24 -78) W/m2 = 66 W/m2 . The ratio of the integrated areas under the curves, if they were plotted on an energy scale would then have to be about 265 / 66 = 4.02. For the moment, let us forget the problem of the abscissa not being linear in energy. We will just multiply the amplitude of the solar insolation curve by a factor of four.
Now let us examine the CO2 absorption plot by itself. Observe the four most intense peaks and note that if we have multiplied the amplitude of the solar insolation curve by four, then the third of the four largest CO2 absorption peaks from the left side has about the same effect on both the solar insolation spectrum and on the surface emission spectrum at about 287.65K. We will amplify the magnitude of the CO2 absorption peaks to its left by a factor of four. Comparing the four-fold increased area of those peaks in the energy range for the solar insolation with those in the surface emission energy range, one finds that the energy absorbed by CO2 from the solar insolation is about 1.3 times that absorbed from the surface emission spectrum. What is more, because the energy ranges on the solar insolation side are compressed and those on the surface emissions side are stretched, this is an under-estimate. CO2 by itself is clearly doing more to cool the surface by keeping solar energy from reaching it, than it absorbs on the emission side.
We also have to remember that even if the energy absorbed from the sun were equal to that absorbed from the surface, the effect would still really be a cooling of the surface. This is because of a built-in asymmetry in the energy transport processes. Contrary to the popular misconception, energy absorption by CO2 from the radiation in the surface emission spectrum does not warm the surface as we have discussed. This absorbed energy is doomed to follow the same path as the energy absorbed by the atmosphere out of the incoming solar insolation. That energy will percolate upward and be emitted from higher up in the atmosphere without affecting the surface temperature.
The Mean Atmospheric Radiative Altitude
If
radiative cooling from the surface is a large fraction of the Earth’s total
cooling, one would not expect the space radiative equilibrium temperature of
255K to be found at the upper part of the water high concentration zone and
near the upper end of its 4500 m to 6500 m direct emission range into
space. One would have a weighting of the
water emission altitudes with the surface in which the surface would enjoy an
advantage due to its higher emission temperature and expect a lower top to the
portion of the atmosphere not in radiative equilibrium with space. Consequently, the altitude at 255K would be
lower than that we find it at, namely 5105 m interpolating from the U.S. Standard
Atmosphere Table of 1976.
Indeed, it is interesting to calculate a
the mean altitude from which water vapor would emit most of the Earth’s IR
radiation off into space. We know that
the Earth radiates about 235 W/m2 of IR radiation into space. We also determined that the direct radiation
emitted from the surface into the atmospheric window and which is the only
radiation from the surface seen in space is about 33 W/m2. Subtracting this from the total Earth IR
emission of 235 W/m2 we find that the top of the water vapor layer
emits almost all of the remaining IR radiation into space, which is 202 W/m2. Since the U.S. Standard Atmosphere puts the
altitude with the temperature of 255.0K at about 5105 m, we can calculate the
effective mean IR-emitting gas radiation altitude needed make this so. Let us call this altitude H, then
[(202 W/m2) / (235 W/m2)] H = 5105
m
H = 5939 m
An effective mean IR-emitting gas altitude
for the IR photons emitted into space of 5939 m seems to be a reasonable
value. If it is, then we can understand
why the mean radiative weighted between direct surface IR radiation and the
IR-active gas radiation is about 5105 m.
Surface Absorption of Back Radiation
Real materials on the Earth's surface do not absorb all infra-red radiation in the mid and long wavelength range equally or with 100% absorption as imagined by the K-T Energy Budget. If they did, FTIR spectroscopy would not be the powerful laboratory spectroscopy that it is for identifying many different materials based upon their widely differing responses in absorbing infrared radiation of different wavelengths. If the actual materials on the surface of the Earth absorbed as black body radiators do, there would be no peaks in the absorption spectra such as will be seen in the materials spectra to be shown. The spectra of absorption would be very uninteresting and be just a long gentle curve across the entire spectrum and absorption levels would be very much higher.
Let us consider some infrared absorption spectra of materials found on the surface of the Earth and compare them to those of water vapor and carbon dioxide to see another reason why the surface does not absorb all of the mid and far infrared radiation incident upon it from the atmosphere and why it is better at absorbing the emissions of water vapor than the emissions of CO2. Most of the Earth’s surface (71%) is covered with liquid water. Water does a pretty good job of absorbing IR radiation emitted by water vapor, since the emitter and the absorber are well-matched in their emission and absorption wavelengths. Minerals and soils on land often are moist or have waters of hydration within the crystal structure of included inorganic compounds. Plants are full of water. As we will see, the same cannot be said surface materials with respect to CO2.
Surface Absorption of Back Radiation
Real materials on the Earth's surface do not absorb all infra-red radiation in the mid and long wavelength range equally or with 100% absorption as imagined by the K-T Energy Budget. If they did, FTIR spectroscopy would not be the powerful laboratory spectroscopy that it is for identifying many different materials based upon their widely differing responses in absorbing infrared radiation of different wavelengths. If the actual materials on the surface of the Earth absorbed as black body radiators do, there would be no peaks in the absorption spectra such as will be seen in the materials spectra to be shown. The spectra of absorption would be very uninteresting and be just a long gentle curve across the entire spectrum and absorption levels would be very much higher.
Let us consider some infrared absorption spectra of materials found on the surface of the Earth and compare them to those of water vapor and carbon dioxide to see another reason why the surface does not absorb all of the mid and far infrared radiation incident upon it from the atmosphere and why it is better at absorbing the emissions of water vapor than the emissions of CO2. Most of the Earth’s surface (71%) is covered with liquid water. Water does a pretty good job of absorbing IR radiation emitted by water vapor, since the emitter and the absorber are well-matched in their emission and absorption wavelengths. Minerals and soils on land often are moist or have waters of hydration within the crystal structure of included inorganic compounds. Plants are full of water. As we will see, the same cannot be said surface materials with respect to CO2.
Fig. 7. The absorption
spectrum of a pool of tap water is shown here taken on an FTIR instrument at 4 cm-1
resolution using the attenuated total reflectance mode. Liquid water absorbs IR radiation at certain
wavelengths capable of exciting vibrational modes in the bonds of its molecule. The spectrum above of absorption in a thin layer of water is clearly nothing like that of a black body absorber. In a deep body of water, the fact that below 3700 cm-1 wavenumbers the absorption does not return to zero allows considerable absorption to occur in the first ten meters or so of the water body. Real bodies of water commonly also have many scattering particulates in them, so some IR is also scattered off such particulates back to the atmosphere. Furthermore, the water temperature commonly drops with depth at a rate that commonly exceeds the drop in temperature with altitude in the air just above the water surface. This means that IR absorbed in the several meters beneath the surface cannot be absorbed by the warmer air a couple of meters above the water surface. Note that 4000 cm-1 is 2.5 µm,
2000 cm-1 is 5 µm, 1000 cm-1 is 10 µm, and 400 cm-1
is 25 µm. Divide 10,000 by the
wavenumber to get the wavelength in micrometers. The water peaks here are at 3300 cm-1
(3.0 µm or 3000 nm) and at 1634 cm-1 (6.1 µm). The broad peak at 3.0 µm is in the very low
energy tail of the Earth’s emission spectrum, while the peak at 6.1 µm is near
the peak in the Earth’s emission spectrum, but not nearly as wide as the water
vapor absorption peak claimed in Fig. 6.
Beyond 25 µm in the Earth’s surface emission spectrum water vapor absorption
in the atmosphere or in much of the Earth’s surface is commonly quite complete. Beyond 25 µm of the Earth’s surface emission
spectrum, one is in the low energy tail of that spectrum. Note that water does not absorb IR radiation
emitted by water vapor with total efficiency.
The peak at 3300 cm-1 is absorbing about 53% of the radiation
incident on it at its peak, while that at 1634 cm-1 is absorbing 34%
of the incident radiation at its peak.
These values may not be the same for back radiation, but they also will
not be totally efficient absorption.
In comparison, the spectrum of CO2 at a
concentration in air many, many times that of the atmosphere is shown here in
transmission mode in the upper spectrum of Fig. 8. The lower spectrum of Fig. 8 shows the effect
of increasing the concentration of CO2 many times beyond the
saturation of the main absorption peak so that a couple of weak absorption
peaks can be seen clearly. Now note that
the range of wavelengths over which CO2 absorbs infrared radiation
is much more limited than the range over which water does.
Fig. 8. The absorption spectrum of CO2 at many times the concentration of the atmosphere is shown. The carbon dioxide concentration in the lower image is much higher than that in the upper image. Note that there is little absorption in the water spectrum where the main CO2 absorption doublet peak at about 2345 cm-1 (4.26 µm) is. Much weaker absorption and emission peaks are found at 3723, 3614, and 664 cm-1 or at 2.69, 2.77, 15.06 µm where the last is the most significant in the low temperature emission spectrum of the Earth. This weaker, but important absorption peak, corresponds to the rising edge of the very long wavelength continuum of water absorption. Water vapor absorption is not commonly saturated at this wavelength between the ground and space, so this is where CO2 is supposed to have its primary effects as a greenhouse gas. It is also the emission peak energy at which water in the surface of the Earth will primarily absorb energy emitted by CO2 molecules in the air. The weak features in the lower partial pressure spectrum of CO2 which do not enlarge in the higher pressure spectrum are likely due to the lowered ratio of CO2 to water vapor in the analyzed air path. This is likely because of dimers or trimers of CO2 and water molecules in complexes. This is not surprising given that such complexes are found in the spaces of interlamellar lattice structures in many minerals.
Of course, much of the land surface is covered by
vegetation, soil, and minerals. Let us
examine a few sample spectra for such materials.
Fig. 9. The infrared
absorption spectrum of a green grass blade is shown. Note that the blade is full of water whose
characteristic peaks are readily seen.
Consequently the blade absorbs radiation emitted by water vapor well,
but note that there is little absorption where the main peaks of CO2
infrared emission are at about 2345 cm-1, so the grass blade is a
comparatively inefficient absorber at that wavelength. It is a better absorber at 664 cm-1
or 15.1 µm.
Fig. 10. The infrared absorption spectra of a green bush
leaf (upper) and a very brown fallen oak leaf (bottom) are shown. The green leaf absorbs IR from water vapor
better than the brown leaf, but both absorb that IR radiation much better than
they do that at the wavelength of the most characteristic CO2
emission. The 15.06 µm CO2
absorption more excited by the Earth’s emission spectrum, will be fairly well
absorbed, but with much less than 100% efficiency.
Fig. 11. The infrared absorption spectrum of a moist and fairly rich soil is shown in the upper image and that of dry sand is shown in the lower image. The moist soil absorbs water vapor IR emissions much better than carbon dioxide IR emission. The dry sand does not absorb either water vapor or carbon dioxide emissions well, except for part of the long wavelength water vapor emission spectrum.
Fig. 12. From top to
bottom, outer bark of an old oak tree, aluminosilicate mineral, feldspar
mineral, and lime mineral infrared absorption spectra. The bark absorbs water vapor emissions well
because it is full of water. The
aluminosilicate is a lamellar material that has water molecules between between
the layers of Si, Al, and O atoms, so it has a slight absorption capability for
water vapor emissions, most easily seen in the broad peak at about 3300 cm-1. Neither the feldspar nor the lime are very
good absorbers for water vapor emissions, though they absorb somewhat at the long
wavelength end of the characteristic water spectrum. None of these materials is an efficient CO2
IR absorber.
Those portions of the Earth covered with water, wet or moist
with water, and covered with life, will have a substantial ability to absorb IR
radiation from water vapor in the atmosphere.
Areas covered with relatively dry minerals will generally not absorb
such water vapor IR emissions well.
Generally, the emissions from CO2 molecules are significantly
less well absorbed by the materials covering the Earth’s surface than those of water vapor. That fraction of the 15.8 W/m2
upper bound on IR radiation that may be incident upon the surface and absorbed which
is due to water vapor emissions is generally going to be absorbed with a higher
efficiency than will that part due to carbon dioxide molecule emissions.
We see that the absorption spectra of real materials of the Earth's surface show that they do not absorb IR radiation in the wavelengths emitted by a real black body radiator at 288K as a black body would. The absorptions would not show peaks, but only a broad curve across the entire spectrum if these materials behaved as black body absorbers do. If they do not behave as black body like absorbers, then they should not act as black body radiators. According to Kirchoff's Law, the absorptivity and the emissivity of a black body like radiator must be equal. It therefore should not be surprising that the effective emissivity that we calculated for the Earth's surface was about 0.5, rather than a value near 1, which a black body would have.
As we have seen, the upper limit on the amount of back radiation is low, especially when compared to the extremely hyped value of the K-T energy budget of Figure 2. Realistically, the back radiation is much lower than the upper limit. Given the usual temperature differential over a mean free path for absorption in the bottom 4 km of the atmosphere, the amount of energy transported in the upward direction by radiation in most cases is very small. Temperature inversions do occur and not too infrequently. Sometimes this allows a net flow of energy downward, but not usually. We have seen the absorption spectra of many of the materials found on the Earth's surface and they cannot absorb all of the energy that is incident. That energy must be reflected. It will soon be re-absorbed by IR-active molecules in the atmosphere.
The Net Cooling Effect of So-Called Greenhouse Gases
Variations in water vapor concentrations in the atmosphere are not only more important than those of CO2 because there is so much more water vapor than CO2, but also because much, much more of the Earth’s surface has a much higher IR absorption efficiency for water vapor emissions than for carbon dioxide emissions. The high preference of surface absorption for IR emissions from water vapor compared to that from CO2 is not recognized in most accounts of how the greenhouse effect is supposed to work based upon back-radiation and how man’s use of fossil fuels is supposed to result in catastrophic warming.
We see that the absorption spectra of real materials of the Earth's surface show that they do not absorb IR radiation in the wavelengths emitted by a real black body radiator at 288K as a black body would. The absorptions would not show peaks, but only a broad curve across the entire spectrum if these materials behaved as black body absorbers do. If they do not behave as black body like absorbers, then they should not act as black body radiators. According to Kirchoff's Law, the absorptivity and the emissivity of a black body like radiator must be equal. It therefore should not be surprising that the effective emissivity that we calculated for the Earth's surface was about 0.5, rather than a value near 1, which a black body would have.
As we have seen, the upper limit on the amount of back radiation is low, especially when compared to the extremely hyped value of the K-T energy budget of Figure 2. Realistically, the back radiation is much lower than the upper limit. Given the usual temperature differential over a mean free path for absorption in the bottom 4 km of the atmosphere, the amount of energy transported in the upward direction by radiation in most cases is very small. Temperature inversions do occur and not too infrequently. Sometimes this allows a net flow of energy downward, but not usually. We have seen the absorption spectra of many of the materials found on the Earth's surface and they cannot absorb all of the energy that is incident. That energy must be reflected. It will soon be re-absorbed by IR-active molecules in the atmosphere.
The Net Cooling Effect of So-Called Greenhouse Gases
Variations in water vapor concentrations in the atmosphere are not only more important than those of CO2 because there is so much more water vapor than CO2, but also because much, much more of the Earth’s surface has a much higher IR absorption efficiency for water vapor emissions than for carbon dioxide emissions. The high preference of surface absorption for IR emissions from water vapor compared to that from CO2 is not recognized in most accounts of how the greenhouse effect is supposed to work based upon back-radiation and how man’s use of fossil fuels is supposed to result in catastrophic warming.
Let us recall that the near infrared absorption of the
atmosphere of solar insolation due to water vapor and carbon dioxide was about 65.0
W/m2. This was energy which the IR-active gases have kept from reaching the surface directly and warming it. Compare this to the
upper limit of 15.8 W/m2 for
mid and far infrared back-radiation incident upon the surface from greenhouse
gases in the atmosphere. It is clear that the net effect of the IR-active
gases in the lower troposphere not in radiative equilibrium with space is a cooling effect! Since mid and far infrared surface absorption
is not totally efficient and we already have reason to believe that this upper
limit is a generous upper limit, this cooling effect is significantly larger
than the 49.2 W/m2 difference between these numbers.
To be sure, this does not separately address the effect of additional carbon dioxide in several respects. First, the atmospheric absorption shielding of the surface from solar insolation does not separate out carbon dioxide from water vapor or ozone absorption effects. Second, we do not have data on the extent to which the effect of added carbon dioxide shielding is saturated versus the degree of saturation with respect to any back radiation effect. Both are near saturation, but is there just enough imbalance in the degree of saturation that added CO2 will create some small shift in the blocking versus the back radiation effects for that added amount. Insofar as a positive feedback of added warming due to water vapor is invoked to add to the miniscule CO2 effect even when that effect is highly exaggerated, it seems clear that added water vapor is not a highly saturated effect in terms of blocking incoming solar radiation. The overall blocking versus back-radiation power densities make it pretty likely that if added CO2 increased the temperature and increased water vapor, then the water vapor increase will provide offsetting cooling. The water vapor feedback is surely negative rather than positive as required by the IPCC to claim a significant warming effect due to added CO2.
To be sure, this does not separately address the effect of additional carbon dioxide in several respects. First, the atmospheric absorption shielding of the surface from solar insolation does not separate out carbon dioxide from water vapor or ozone absorption effects. Second, we do not have data on the extent to which the effect of added carbon dioxide shielding is saturated versus the degree of saturation with respect to any back radiation effect. Both are near saturation, but is there just enough imbalance in the degree of saturation that added CO2 will create some small shift in the blocking versus the back radiation effects for that added amount. Insofar as a positive feedback of added warming due to water vapor is invoked to add to the miniscule CO2 effect even when that effect is highly exaggerated, it seems clear that added water vapor is not a highly saturated effect in terms of blocking incoming solar radiation. The overall blocking versus back-radiation power densities make it pretty likely that if added CO2 increased the temperature and increased water vapor, then the water vapor increase will provide offsetting cooling. The water vapor feedback is surely negative rather than positive as required by the IPCC to claim a significant warming effect due to added CO2.
What we have found is that the picture of a large back
radiation warming of the Earth’s surface as given by the Kiehl-Trenberth energy
budget diagram of Fig. 2 is a very unphysical and wrong view of the real energy
budget. If there is any significant warming
of the Earth’s surface due to having an increase in the concentration of carbon
dioxide, it is not due to increased back radiation. It is certain that the effect of increased
water vapor in the lower atmosphere is actually a cooling effect during the
daily cycle, though increased water vapor can provide a decrease of night-time
cooling, thereby being a warming effect at night. But, due to the failure of the Earth’s
surface to absorb the IR emission of CO2 as readily as that of water
vapor, it is clear that an increase in CO2 will not have as large a warming
effect at night as does water vapor on a per molecule basis.
Discussion of Added Carbon Dioxide Effects in the Upper Troposphere and the Tropopause
Discussion of Added Carbon Dioxide Effects in the Upper Troposphere and the Tropopause
One way an increase in the concentration of CO2 in the
atmosphere may have a warming effect on the Earth’s surface is to move the altitude of effective radiative equilibrium with space outward
so that the gravitational temperature gradient in the atmosphere operates over a longer range so the surface temperature is raised. It would have to do
this by increasing the net radiative cooling at high altitudes. Thus even neglecting
the negative feedback responses to moving the equilibrium shell outward, an
increase in temperature at the Earth’s surface of 1 K or 1ºC would require an
increase in the CO2 concentration to increase the effective shell
altitude by 154 meters given the static equilibrium temperature
gradient of 6.49K/km. Actually more
because increased CO2 would also decrease the solar insolation
directly absorbed at the surface or at the top of the water vapor IR emission
layer.
The Earth’s surface emits radiation into space directly through the atmospheric window. Water vapor is most prevalent at altitudes below its freezing temperature, which occurs at the altitude of about 2300 m. Yet there is enough water vapor above this altitude that almost all of its emission of IR radiation into space from the atmosphere is from altitudes of about 2500 to 6000 m, so this majority IR-emitter emits at much warmer temperatures than does the relatively rare CO2 molecule, which emits from altitudes of about 9,000 to 20,000 m. Because of its lower radiative temperature, its smaller characteristic frequency ranges, and its rarity, CO2 provides a relatively small portion of the radiative cooling of the planet as a whole. Water vapor is the dominant greenhouse gas molecule by virtue of its much greater concentration, its shorter re-emission time, its wider range of absorption and emission frequencies, and its tendency to form dimers and trimers with other atoms or molecules to give it a still wider range of absorption and emission frequencies.
The Earth’s surface emits radiation into space directly through the atmospheric window. Water vapor is most prevalent at altitudes below its freezing temperature, which occurs at the altitude of about 2300 m. Yet there is enough water vapor above this altitude that almost all of its emission of IR radiation into space from the atmosphere is from altitudes of about 2500 to 6000 m, so this majority IR-emitter emits at much warmer temperatures than does the relatively rare CO2 molecule, which emits from altitudes of about 9,000 to 20,000 m. Because of its lower radiative temperature, its smaller characteristic frequency ranges, and its rarity, CO2 provides a relatively small portion of the radiative cooling of the planet as a whole. Water vapor is the dominant greenhouse gas molecule by virtue of its much greater concentration, its shorter re-emission time, its wider range of absorption and emission frequencies, and its tendency to form dimers and trimers with other atoms or molecules to give it a still wider range of absorption and emission frequencies.
There are also negative feedbacks to added CO2 causing
the effective radiative balance point to effectively move to higher
altitudes. One of these is the fact that
the important issue with respect to the gravity induced temperature gradient is
where do fast radiative heat transfer mechanisms become dominant over the slow
heat transport mechanisms of evaporation and water transport, air conduction
and convection, and winds. This altitude
is already determined by water vapor.
Adding CO2 at altitudes above water vapor’s emission
altitudes, simply has very little effect on the net rate of radiative heat
transport. CO2 mostly absorbs
radiation where water vapor does and just relays it along. Of course, any decrease of radiation into space is counteracted by the increase in the numbers of CO2 emitters and by any warming of the atmosphere from which it emits due to any decreased radiation cooling efficiency by CO2. There will be whatever adjustments are needed to maintain the radiative equilibrium with space. To date, increasing CO2 concentrations have not produced measurable temperature increases at 8 to 12 km altitude. This is actually evidence that there is no effect of decreased radiation into space due to higher CO2 concentrations.
Consequently, an argument based on the gravitational temperature gradient will not work. What is more, one has to allow that more CO2 in the upper atmosphere should mean more absorption of the IR portion of the incoming solar insolation and that is surely a cooling effect upon surface temperatures.
Consequently, an argument based on the gravitational temperature gradient will not work. What is more, one has to allow that more CO2 in the upper atmosphere should mean more absorption of the IR portion of the incoming solar insolation and that is surely a cooling effect upon surface temperatures.
Another version of the idea is that more CO2 at
altitudes of 8 to 12 km would cause CO2 to emit less IR radiation
into space because the zone of atmospheric transparency for the main CO2
emission wavelength would rise to a higher altitude of cooler gas. The radiative efficiency of the CO2
would decrease and the entire atmosphere would simply and directly warm up for
that reason. This version of a high
altitude effect has CO2 serving as a less effective coolant than the
version of the preceding paragraphs.
It is claimed that a doubling of the CO2 concentration will cause an increase in the surface temperature of 1.2 K due to a decrease in the radiative cooling of the atmosphere of 3.7 W/m2. This cooling decrease is based on the foolish assumption that all of the surface warming since the end of the Little Ice Age is due to an increase in the concentration of CO2 in the atmosphere. Note that the 1.2K increase due to doubling the CO2 concentration is that predicted due to CO2 increasing alone and does not include the IPCC prediction of a total 5.4K increase due mostly to a positive feedback due to increased water vapor, for which there is no evidence either.
It is claimed that a doubling of the CO2 concentration will cause an increase in the surface temperature of 1.2 K due to a decrease in the radiative cooling of the atmosphere of 3.7 W/m2. This cooling decrease is based on the foolish assumption that all of the surface warming since the end of the Little Ice Age is due to an increase in the concentration of CO2 in the atmosphere. Note that the 1.2K increase due to doubling the CO2 concentration is that predicted due to CO2 increasing alone and does not include the IPCC prediction of a total 5.4K increase due mostly to a positive feedback due to increased water vapor, for which there is no evidence either.
The general circulation climate models predict a slight
warming of the 8 to 12 km altitudes over the Equator and the lower latitudes,
but this has not been found to be the case.
There is as yet no clear evidence that increasing CO2 is
actually re-structuring the atmosphere in such a way as to significantly move
the shell of effective radiative equilibrium with space or to change the overall
temperature of the upper troposphere.
However, satellite measurements of Earth emission data shows
that the IR photons absorbed by CO2 molecules are not re-emitted
before the energy gained is redistributed by collisions with other
non-greenhouse gas molecules. We know
this because the re-emission of radiation does not occur at a black body
temperature of 288K and instead occurs at a black body temperature of about 210
to 220K characteristic of general air temperatures at altitudes from 10.5 km to
22 km. [See Fig. 8.3 of A First Course
in Atmospheric Radiation, First Edition, Grant W. Petty, Sundog Publishing,
Madison, Wisconsin for such measured spectra and observe the CO2
absorption region of 15 micrometers wavelength.
This is a weaker emission line than the primary emission line, so it is
less subject to saturation at a lower altitude.
The main emission line will not have its final emissions into space from
a lower altitude, but will be higher if anything. There is a similar spectrum in Fig. 25 of the
Ermecke paper.]
This is an important contradiction of the claim that CO2 emission into space is primarily from 8 to 9 km in altitude as is sometimes claimed. Because from 11 km to 20 km the temperature is almost constant at 217K, this being the tropopause, more radiation from this altitude is not important from the standpoint of moving the overall altitude of effective radiative equilibrium with space. The supposition that increasing the CO2 concentration will cause CO2 emitters to emit at a lower temperature into space and decrease the overall radiative cooling is wrong.
This is an important contradiction of the claim that CO2 emission into space is primarily from 8 to 9 km in altitude as is sometimes claimed. Because from 11 km to 20 km the temperature is almost constant at 217K, this being the tropopause, more radiation from this altitude is not important from the standpoint of moving the overall altitude of effective radiative equilibrium with space. The supposition that increasing the CO2 concentration will cause CO2 emitters to emit at a lower temperature into space and decrease the overall radiative cooling is wrong.
One way or
another, the planet as a whole has to be in radiative equilibrium with
space. As long as the radiative energy
inputs of space do not change, the radiative response of the Earth must equal
that input. As we have seen, the surface temperature is determined by the absorbed solar insolation and the range over which non-radiative energy transport plays a major role. If there is a decrease in
the radiative output of CO2 in our atmosphere at altitudes higher than those in which other transport mechanisms are important, then the net
radiation output of other IR-emitting molecules must increase to compensate for
that decrease attributed to CO2.
Since water is the dominant IR-emitter, any change in the CO2
power emission into space will invoke an equal and negative feedback from water
vapor.
Note that such a negative feedback need not apply in the near surface atmosphere where the atmosphere is not in radiative equilibrium with space. It must apply to the Earth’s radiation into space, barring a small caveat for other energy couplings with space such as the solar wind, debris entering our atmosphere, and couplings of the Earth’s magnetic field to the sun’s magnetic field. The small heat flow from the Earth’s deep interior is also another small, but genuine, heat source. However, the sun is by far the dominant and controlling heat source and the Earth is for most practical purposes simply in a radiative equilibrium, albeit over a substantial period of time due the great heat capacity of the oceans, the Earth’s land surface, and to a lesser extent the atmosphere.
Conclusions
Note that such a negative feedback need not apply in the near surface atmosphere where the atmosphere is not in radiative equilibrium with space. It must apply to the Earth’s radiation into space, barring a small caveat for other energy couplings with space such as the solar wind, debris entering our atmosphere, and couplings of the Earth’s magnetic field to the sun’s magnetic field. The small heat flow from the Earth’s deep interior is also another small, but genuine, heat source. However, the sun is by far the dominant and controlling heat source and the Earth is for most practical purposes simply in a radiative equilibrium, albeit over a substantial period of time due the great heat capacity of the oceans, the Earth’s land surface, and to a lesser extent the atmosphere.
Conclusions
The bottom line is this:
There is no reason to believe that increased CO2 in the
Earth’s atmosphere, whether due to man’s use of fossil fuels or a general
decrease in its solubility in oceans still slowly warming since the end of the
Little Ice Age, will cause an increase in the surface temperature of the
Earth. In fact, it is more likely to
cause a small decrease in the surface temperature due to increased atmospheric
absorption of incoming solar insolation in the near infrared.
It is clear that the net effect of the IR-absorbing gases now in our lower atmosphere is a surface cooling effect, yet is also true that without water vapor in our atmosphere and a dense lower atmosphere of infra-red inactive gases, the necessary conditions would not exist to keep the Earth’s surface from being in radiative equilibrium with space. This would mean that the surface temperature would be much cooler on average with disastrous temperature cycles during the daily cycle. Actually so much would be different that the surface temperature of the Earth would behave much like that of the moon. Thus it is correct to say that IR-active water vapor warms the Earth’s surface as an essential part of the complex mechanism that allows the surface to be substantially warmer than it would be in direct radiative equilibrium with space.
Yet, it is very important to know the context in which this is true and to understand that carbon dioxide does not have the strong effects of water vapor. In fact, it is probably a coolant in all respects. Water and water vapor act as coolants and warming agents within the framework of an Earth covered with water and surrounded by a thick, predominantly IR-inactive atmosphere of gases. Their roles are complex and fortunately act within a very reliable and stable set of feedbacks that moderate changes in the Earth's surface temperature.
Increased carbon dioxide concentrations in the atmosphere are actually good for plants and all the animals that rely on plants. Most plants evolved under conditions of much higher carbon dioxide concentrations in the atmosphere and thrive with more of the carbon dioxide that is essential food for them. Greenhouse operators have long greatly increased the carbon dioxide concentrations inside their greenhouses to get very substantial increases in plant growth, flowering, and fruit production.
Professor Cliff Ollier has presented an excellent discussion of the effects of added atmospheric carbon dioxide upon plants and animals of the oceans. Marine animals such as coral and shellfish that use carbon dioxide for protective housing thrive with higher concentrations of carbon dioxide. The claim that such higher concentrations of atmospheric carbon dioxide will cause the oceans to become acidic is false. Coral and shellfish have actually been so effective in converting carbon dioxide into limestone sediments over the eons that they are responsible for the Earth having too little atmospheric carbon dioxide now for the good of plants and animals.
It is clear that the net effect of the IR-absorbing gases now in our lower atmosphere is a surface cooling effect, yet is also true that without water vapor in our atmosphere and a dense lower atmosphere of infra-red inactive gases, the necessary conditions would not exist to keep the Earth’s surface from being in radiative equilibrium with space. This would mean that the surface temperature would be much cooler on average with disastrous temperature cycles during the daily cycle. Actually so much would be different that the surface temperature of the Earth would behave much like that of the moon. Thus it is correct to say that IR-active water vapor warms the Earth’s surface as an essential part of the complex mechanism that allows the surface to be substantially warmer than it would be in direct radiative equilibrium with space.
Yet, it is very important to know the context in which this is true and to understand that carbon dioxide does not have the strong effects of water vapor. In fact, it is probably a coolant in all respects. Water and water vapor act as coolants and warming agents within the framework of an Earth covered with water and surrounded by a thick, predominantly IR-inactive atmosphere of gases. Their roles are complex and fortunately act within a very reliable and stable set of feedbacks that moderate changes in the Earth's surface temperature.
Increased carbon dioxide concentrations in the atmosphere are actually good for plants and all the animals that rely on plants. Most plants evolved under conditions of much higher carbon dioxide concentrations in the atmosphere and thrive with more of the carbon dioxide that is essential food for them. Greenhouse operators have long greatly increased the carbon dioxide concentrations inside their greenhouses to get very substantial increases in plant growth, flowering, and fruit production.
Professor Cliff Ollier has presented an excellent discussion of the effects of added atmospheric carbon dioxide upon plants and animals of the oceans. Marine animals such as coral and shellfish that use carbon dioxide for protective housing thrive with higher concentrations of carbon dioxide. The claim that such higher concentrations of atmospheric carbon dioxide will cause the oceans to become acidic is false. Coral and shellfish have actually been so effective in converting carbon dioxide into limestone sediments over the eons that they are responsible for the Earth having too little atmospheric carbon dioxide now for the good of plants and animals.
It is also necessary to note that the claim that
increasing CO2 concentrations in the atmosphere mean a catastrophically increasing
surface temperature is based on a very poor understanding of and application of
physics. The fact that so many
professional science organizations have claimed that the catastrophic man-made
global warming hypothesis is now settled science is a disgrace. In addition to the many problems with the physics used to support the hypothesis of catastrophic effects, there is a long history of geological evidence that high CO2 concentrations in the atmosphere do not cause warming and catastrophic conditions that have threatened plants and animals. The evidence is that the climate is fairly stable and much more affected by changes in solar insolation and cosmic ray nucleation of clouds than by CO2 concentrations. There is a predominance of evidence that warming results in increases in the atmospheric CO2, rather than the other way around. Contrary to the suppositions of the catastrophic warming hypothesis, there is even evidence now that the warming since 1982 preceded increases in atmospheric CO2.
There are also many sad instances in which the warm periods of the historical past have been manipulated out of the scientific record. The warm 1930s have been artificial jiggered to cooler temperatures, as has most of the surface temperature data between then and about 1975. Somehow urban heat island effects were more in need of correction when the human population was smaller than it has been in this most recent period back to 1980. Then there is the loss of many rural weather stations since in the surface temperature records and much evidence that temperatures measured by rural stations did not show significant increases. The Medieval Warming and the Roman Warming were all minimized. Proxy temperature data was often manipulated to minimize the temperatures of prior warm periods.
Scientists who have gone along with this theory of catastrophic effects caused by carbon dioxide emissions have been rewarded with over $100 billion of research money by the U.S. Government or additional money from other governments. By giving many politicians more excuses for expanding the role of governments in controlling their people, businesses, resources, and the standard of living of their people, many posing as scientists have become handmaidens to tyranny. Handmaiden is a nice way of saying what these scientists and scientific organizations have really become. This is, of course, a betrayal of science by many who are supposed to be dedicated to its rational, objective, and critical thinking requirements.
This post was first posted on 17 February 2013 and continued to be updated frequently until 7 April 2013. Additional comments were added on 1 June 2014. Still further comments were added on 10 and 12 August 2014 [relating to near-IR infra-red absorption and emission by neutral atoms of N, O, C, and Ar resulting from a comment below by MS]. I added to the section The Black Body and the Earth Radiator before Fig. 2. to clarify why the radiative emissivity of the Earth's surface is about 0.48 or 0.5 and not near 0.95 as so many claim on 20 January 2015. Further minor editing was done on 7 March 2015.
There are also many sad instances in which the warm periods of the historical past have been manipulated out of the scientific record. The warm 1930s have been artificial jiggered to cooler temperatures, as has most of the surface temperature data between then and about 1975. Somehow urban heat island effects were more in need of correction when the human population was smaller than it has been in this most recent period back to 1980. Then there is the loss of many rural weather stations since in the surface temperature records and much evidence that temperatures measured by rural stations did not show significant increases. The Medieval Warming and the Roman Warming were all minimized. Proxy temperature data was often manipulated to minimize the temperatures of prior warm periods.
Scientists who have gone along with this theory of catastrophic effects caused by carbon dioxide emissions have been rewarded with over $100 billion of research money by the U.S. Government or additional money from other governments. By giving many politicians more excuses for expanding the role of governments in controlling their people, businesses, resources, and the standard of living of their people, many posing as scientists have become handmaidens to tyranny. Handmaiden is a nice way of saying what these scientists and scientific organizations have really become. This is, of course, a betrayal of science by many who are supposed to be dedicated to its rational, objective, and critical thinking requirements.
This post was first posted on 17 February 2013 and continued to be updated frequently until 7 April 2013. Additional comments were added on 1 June 2014. Still further comments were added on 10 and 12 August 2014 [relating to near-IR infra-red absorption and emission by neutral atoms of N, O, C, and Ar resulting from a comment below by MS]. I added to the section The Black Body and the Earth Radiator before Fig. 2. to clarify why the radiative emissivity of the Earth's surface is about 0.48 or 0.5 and not near 0.95 as so many claim on 20 January 2015. Further minor editing was done on 7 March 2015.
This paper is available in .pdf format and will be sent upon request. An updated version of this paper is now posted here.









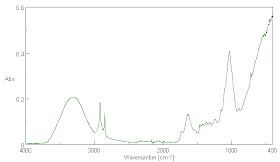






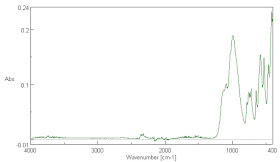

I have a problem with the statement-
ReplyDelete"If it were not, then the radiative equivalent temperature of the Earth as a whole and averaged over the day would be lower than 255K and might be more like the 228K of the moon."
There surely cannot be any doubt that the extreme low temperatures on the Moon are due to the very long "night" of the moon - some 14.5 days or 354 Earth hours - just as the reason for the cold poles on Earth is a combination of low incident solar radiation plus prolonged periods of little to no insolation.
If the Moon had a period of 24 hours I cannot see any reason why it wouldn't be substantially hotter on average that the Earth.
After all from noon til dawn on the Moon is approximately 531 Earth hours and the temperature goes from say 370 K to 85 K.
This represents a rate of cooling of about 0.54 K per hour.
Even from noon to sunset where I have seen figures of 390 K to 220 K quoted the rate of cooling is about 170 K in 177 hours - a maximum of 1 K per hour.
If the Moon had the same period as the Earth after 12 hours of night one could expect the dawn temperature on the Moon to be in the order of 350 K.
To ignore the different periods of the Moon and Earth in comparing radiative effects seems unrealistic to me.
The Earth without an atmosphere and oceans must be significantly hotter just as the Moon with a similar period must be.
If I am mistaken in this please explain - my email for contact is roscomac@dodo.com.au.
You make a good point that the added length of the moon's daily cycle is significant.
ReplyDeleteI changed the paragraph in question to read:
Analogously, the Earth's land surface, its oceans covering 70% of the planet, and its atmosphere all have a heat capacity and provide for a substantial flow of heat to the surface from their interiors at various times of the day. The heat capacities of the Earth’s surface and atmosphere greatly exceed that of the rock of the moon, especially thanks to our oceans, so the day to night moderating effect seen on the Earth is much larger than it is for the moon. It is true, as pointed out by Rosco in a comment, that the moon also achieves both higher and lower temperatures than would the Earth simply because its daily cycle is much longer. Nonetheless, the radiative equivalent temperature of the Earth as a whole and averaged over the day would be lower than 255K if the solar insolation reaching the surface were unchanged because higher daylight temperatures would cause more effective cooling during the day than during the night.
Under the condition of having no IR-absorbing gases in our atmosphere, the Earth's surface temperature would be about 318K as I pointed out in The Earth Surface Temperature without Greenhouse Gases: The Shade Effect of Infra-Red Active Gases, posted on 1 April 2013.
Charles, this is a thought provoking discussion that I need to read a second, and probably third time to take on board all the details. However I'd like to comment on your discussion of temperature gradients in a static atmosphere as a result of gravity. This is a classic problem in physics and one discussed by Feynman in his lectures (volume 1) in which he demonstrates that a static gas in a gravitational field is actually isothermal. The crux of the argument is that whilst the total kinetic energy of an ensemble of molecules decreases with height the velocity distribution actually remains constant and thus the average velocity and temperature does not change.
ReplyDeleteI don't think this impacts in a significant way on your discussion which I now want to go back and read again. Thank you once again for a thought provoking presentation and one that helps me to crystallise some of my own thinking on this issue. You may be interested to know that I am a geochemist working on palaeoclimate reconstructions in deep geological time.
Thank you for your comment Paul. I was not aware of Prof. Richard Feynman's argument on this subject, though I have heard others claim that a static atmosphere should be isothermal. It would be an interesting outcome if the U.S. Standard Atmosphere calculations that went through several versions were performed on such a wrongheaded basis in its own right. My effort here was to produce a simple argument that gave similar results to those calculations.
ReplyDeleteI have Feynman's 3-volume set and have enjoyed reading portions of them over the years. However, I had not read this part of Vol. 1 to which you are referring. The index is not very extensive and it has taken me awhile to even figure out where this argument on his part likely is. Having now identified the likely sections, I will read them and either concede to him or try to explain why I think he is wrong. Imagine that! This is a very busy time at my laboratory, so my addressing this will have to wait for the weekend, which even so will be filled with work.
But, there is no question that Prof. Feynman deserves a thoughtful response!
aldo alesii had problems with the submission of this comment and asked me to post it for him.
ReplyDeleteGreat blog Charles!
First, I want to applaud you for not calling CO2 a "greenhouse gas" and instead referring to it as an infrared-absorbing gas. Free atmospheric CO2 behaves nothing like a greenhouse, which heats up above the ambient temperature due to the prevention of CONVECTION of the gases inside by the structure of the greenhouse. I believe the incorrect use of the "greenhouse" moniker by the warmists is to intentionally implant in the minds of the general population the vision of a hothouse world even before "firing a single shot" so to speak.
Secondly, since the big bone of contention is CO2 and its absorption of some infrared and what happens to its temperature, what do you think would happen if one did the following simple experiment.
Take two greenhouses (two sealed cardboard boxes with the top covered with saran wrap), one filled with air and the other with CO2 and just put them side by side in a room at about 70 degree F. (294K). In other words, the boxes are immersed in a sea of infrared radiations near the absorption peak of CO2.
What would you expect the temperature readings inside the two boxes to be? (even in relative terms).
aldo alesii,
ReplyDeleteOn your question about the saran wrap covered boxes in a 70F room, I am assuming that the radiation flying about the room is on that infrared radiation due to 70F surfaces. Therefore, both boxes are at the equilibrium temperature of 70F. If the experiment you had in mind has some other description, I am missing that information. For instance, are the two boxes sitting in direct sunlight coming through an open window?
I have often seen the claim that Oxygen and Nitrogen do not emit significant quantities of infra-red radiation. I understand some of the reasoning for these claims.
ReplyDeleteKirchhoff's law, the "transparency" of the atmosphere that allows IR detection devices to function etc.
But is it realistic to claim that O2 and N2 do not radiate significant IR ?
there can be no doubt these constituents of our atmosphere increase and decrease in temperature - that is indisputable !
If they do not radiate significant IR then the only mechanism for them to lose energy and thus cool must be the random and infrequent - given the low concentrations involved - collisions between them and "greenhouse gases".
If this is true and K & T 's energy budget has any semblance of reality given they claim almost 83% of the 235 emitted to space comes from the atmosphere which they claim O2 and N2 play no significant role in it seems extremely difficult to understand why increasing the concentration of the substances that transmit 83% of the energy to space will cause warming.
The second "fraud" shown regularly is the 2 curves of blackbody radiation for the Sun and Earth which give the FALSE impression similar energy is emitted by the Earth as the insolation. If true scale were used the terrestrial radiation would be indistinguishable from the "X" axis of the graph.
The third "fraud" is ignoring that CO2 has 3 major wavelength bands for absorption.
The lower wavelength bands correspond to the peak emission of an object at ~1000 K and ~800 K respectively.
The terrestrial radiation is likely to have effectively energy at these wavelengths BUT the solar insolation is likely to have significant energy levels.
Isn't it likely that any portion of the Down-Welling Long-wave Radiation due to CO2 is a direct consequence of absorption from the solar IR and as such reduces the "heating" effect of the solar radiation on the surface ? As it probably is for water vapour ?
Is my reasoning in these somehow flawed - they seem much more believable than the radiation from a "layer" of GHGs at the temperature found in my freezer is capable of heating the Earth's surfaces more than the Sun can ?
Thank you for your comments Rosco.
ReplyDeleteOxygen and nitrogen gas and the atmosphere generally are completely absorbing for very long wavelength microwaves and do emit radiation at such wavelengths. See my post of 18 March 2013 on this: http://objectivistindividualist.blogspot.com/2013/03/do-all-atmospheric-gases-absorb-and.html
I have not seen any quantitative data on the total rate of energy loss of the Earth system due to microwave radiation. This is one of many natural effects never discussed and perhaps poorly characterized. Such microwave photons carry little energy individually, but there are many emitters and the rate of emission is also a variable.
O2 absorbs solar radiation at the boundary of visible and near IR radiation. Presumably, it re-emits half of what it absorbs to space. While the fraction of the solar insolation so absorbed is very small, each photon emitted has an energy much greater than each photon emitted by the Earth's surface. This O2 absorption is always glossed over in discussions and never quantified. While I do not believe the effect is large, it may be significant in comparison to any effect of CO2.
CO2 absorbs a good deal of IR radiation in the solar insolation spectrum between 1.4 and 3 microns wavelength, so adding CO2 reduces the amount of solar energy reaching the Earth's surface. See Figs. 3 and 4. I believe this solar insolation absorption effect is comparable to any CO2 warming effect upon the surface, thus reducing any net effect on surface temperatures.
Actually collision rates in the troposphere are very high at 6.9 billion per second at sea level, so that even though CO2 molecules are rare and they absorb IR radiation from solar insolation and from the Earth's surface sufficient to raise their temperature, they usually have a collision or several collisions with other atmospheric molecules before they can even re-emit that absorbed IR radiation.
The K-T Earth Energy Budget diagrams are all nonsense. They grossly violate energy conservation. Note that in Fig. 2. with the solar insolation at the top of the atmosphere taken at 100%, 22.5% of solar insolation is said to be reflected from clouds and another 8.8% is reflected from the surface. This only leaves 68.7% of solar insolation anywhere in the thermal energy of the atmosphere plus the Earth's surface. If only 68.7% of the energy is available anywhere, how can 94.7% be back-reflected? Obviously, back-reflection cannot possibly be larger than 68.7%. Indeed, the sum of back-reflection energy, surface IR emission energy, energy into water evaporation, and energy into conduction/convection cannot be greater than 68.7%. Yet that diagram claims their sum is 226.8%. Worse yet, this assumes that none of the 19.6% of the solar insolation absorbed by the atmosphere before it reaches the surface is directly re-emitted to space!
Clearly the K-T diagrams are nonsense. They are energy multipliers in defiance of the Law of Energy Conservation. The actual amount of back-radiation absorbed by the surface is about 0% or barely above that.
Hi Dr. Anderson,
ReplyDeleteI'd be interested in your comments on this since you're an expert in spectroscopy.
A commenter asked today... how do you know that argon is not a greenhouse gas? NIST document on infrared spectra of noble gases, http://nvlpubs.nist.gov/nistpubs/jres/049/2/v49.n02.a04.pdf, lists a rich collection of IR lines of argon.
http://hockeyschtick.blogspot.com/2014/08/paper-proves-bill-nyes-faked-greenhouse.html?showComment=1407606557301#c9211627987147538559
Indeed it does, and the NIST website lists 135 lines in the IR region. This was surprising to me since I thought the monoatomic Noble gases did not absorb/emit in the IR.
Why wouldn't Argon also be called a "greenhouse gas"? Do you know of any plots comparing transmission spectra showing % transmission of Ar vs the "greenhouse gases"?
Since there's more than 23X more Ar in the atmosphere than CO2 I'm wondering if Ar could have a radiative effect on the atmosphere?
Thanks much in advance for your help & kind regards
This is an interesting comment. It caused me to do something I should have done long ago. I simply looked at the Line Spectra of the Elements in my 71st Edition of the Handbook of Chemistry and Physics and found that it listed 84 electronic transitions in neutral argon atoms in the near infra-red range from 750 nm to 3000 nm. The argon emission lines listed range from 750.3869 nm to 2,396.652 nm. Similarly, nitrogen atoms have 55 electronic transition emission lines in the near IR ranging from 739.864 nm to 1,787.826 nm. Oxygen atoms have 87 electronic emission lines ranging from 770.675 nm to 2,617.356 nm. Carbon atoms have a fairly rich emission spectrum also in the near infra-red with 39 emission lines ranging from 786.089 nm to 1,972.199 nm. The stronger argon emission lines in the near IR are more intense than those of nitrogen, oxygen, and carbon, but all of them have rich emission spectra, which means they also have rich absorption spectra.
ReplyDeleteThe arguments that are generally made that argon, nitrogen, and oxygen are not active infr-red absorbing gases are based on vibrational spectra considerations and do not consider electronic transition spectra. Clearly there has been a failure to see the Big Picture here.
The absorption of radiation in the near infra-red will almost entirely be realized as the absorption of solar insolation. I do not have comparative spectra of the electronic transition spectra with the vibrational spectra with respect to intensities.
Thanks for your reply!
ReplyDeleteYes, the Ar absorption & emission is mostly within the solar spectrum
http://www.azimuthproject.org/azimuth/files/earth_and_sun_emission.jpg
I'm wondering given Ar is the 3rd most prevalent atmospheric gas, absorbs in the more energetic near IR vs. "greenhouse gases" whether a significant portion of the so-called GHE is due to solar absorption by Ar?
Because the electronic transition absorptions are in the near infra-red spectrum their effect in argon, carbon, oxygen, and nitrogen atoms is to intercept solar insolation before it reaches the surface of the Earth. The effect is to warm the atmosphere well above the surface level and to cool the surface. I believe I have listed these four atoms in the order of their absorption strength per atom. So argon is a stronger electronic transition absorber than carbon, which is stronger than oxygen, which is stronger than nitrogen. Because this near infra-red absorption is much stronger for each argon atom than for each carbon or oxygen atom and there are many more argon atoms than atoms in CO2 in the atmosphere, this near infra-red absorption effect in argon is much greater than for carbon dioxide.
ReplyDeleteBecause we are mostly interested in the surface temperature, these near infra-red absorption effects would not normally be called a greenhouse effect by many. One thing people are often careless about however is this issue of changes in the distribution of heat in our atmosphere due to the many effects that influence that distribution. From early on, I have tried to draw attention to the many ways water vapor, carbon dioxide, and oxygen adsorb in-coming solar insolation in the atmosphere and thereby diminish the heating of the Earth's surface. I thank you for drawing my attention to the effect of argon and to a better understanding of the near infra-red absorptions in general!
Please note that my response to Rosco's earlier question about infra-red radiation by nitrogen and oxygen is also affected by these near infra-red electronic transition absorptions of incoming solar radiation and their re-emission into space from the atmosphere.
ReplyDeleteSo much interesting information here thanks for sharing
ReplyDeleteWell, I'm interested that you estimate IR emissivity of 0.479 for Earth's surface. Especially in light of the fact that everyone who has actually measured it (different surfaces close up and in the lab, broad swaths of Earth by instruments in airplanes, balloons, and satellites, etc.) gets figures between 0.95 and almost 1.0. What do you suppose explains that discrepancy?
ReplyDeleteI have added to the discussion of this in the section The Black Body and the Earth Radiator before Fig. 2, the Kiehl-Trenberth Earth Energy Budget in the general hope of making my reasoning more clear. Many people who come from the general climate camp seem to be troubled by an effective emissivity of 0.48 rather than 0.95. The emissivity in air should not be the same as the emissivity in vacuum. But, it is also true that one cannot actually measure the emissivity of water as a liguid surface in vacuum also. The vacuum interface would result in rapid cooling of the water surface and wreck havoc on the measurement.
ReplyDeleteBut, as I clearly show, the empirical average value of the emissivity of the Earth's surface at the interface with the atmosphere is effectively about 0.48, unless you want to argue for the overthrow of the Law of Energy Conservation.
I've enjoyed your article and addressed many unanswered questions.
ReplyDeleteRegarding the CAGW theory and the warming oceans. It would appear that the IR spectrum of liquid water is transparent to IR emitted from CO2 given the graphs shown. It would seem unreasonable to assume CO2 could have any direct impact on warming the oceans given this fact. Your thoughts.
The second point is a post on scienceofdoom.com that tries to minimize the CO2 absorption of incoming solar IR. Do you have a moment to point out the error in his logic?
Thanks once more.
CO2 in the Solar Spectrum
Thanks for your comment Doug.
ReplyDeleteBelow 3700 cm-1 the absorption spectrum of liquid water is not zero, though it is low where CO2 has its main emission peak. So water can absorb some IR radiated from a CO2 molecule in the first few meters in the atmosphere above the surface since the water a few meters below the surface of the water body may be colder than the air-borne CO2 molecule. This is an effect of CO2 back-radiation I have not previously given consideration. While this is not likely a trivial effect, I am quite sure that it is not a large enough effect to save the argument for catastrophic man-made global warming. If it were such a savior, presumably the great government-funded scientists after nearly $200 billion of funding would have explained this to us.
Doug, to reply to the Science of Doom argument, we have to remember that the total absorption of the atmosphere of incoming solar insolation counts to prevent radiation from being absorbed by the surface. If our focus is on the surface temperature, as it should be for most of our human concerns, the absorption by CO2 of solar insolation occurs through 70 km of atmosphere. On the other hand, a fact neglected by proponents of catastrophic AGW, the warming effects of CO2 are almost entirely due to those CO2 molecules within a couple of mean free paths of the surface. This is surely less than 100 m. So a much weaker absorption per meter of atmosphere traversed is largely offset by a much longer effective distance over which the cooling effects manifest compared to the warming effects.
ReplyDeleteAnother substantial problem comes with the assumption that the total so-called greenhouse gas effect is a 33K warming of the surface. This is not so and the exaggeration is huge.
Another problem with his argument is that much of the effect of CO2 is said by the catastrophic AGW people to be due to increased water vapor as a result of increased CO2. This water vapor is supposed to amplify greatly the weak CO2 warming effect. Now aside from the fact that many now think increased water vapor produces a cooling effect or no amplification, the Climate of Doom argument would have to repeat the same argument for water vapor as he gave for CO2 in terms of its absorption of solar insolation compared to its back-radiation warming effects. If the water vapor argument that its effects on absorbing solar radiation was comparable to its so-called greenhouse effect, then it would fail to amplify the claimed weak CO2 warming effect. What is more, my first argument on the relevant travel length being the entire atmosphere in the cooling argument versus the mean free path length of only about a meter or so for water vapor would still apply to the water vapor calculation.
I have to agree with the other comment querying as to why wouldn't Argon also be called a "greenhouse gas"?
ReplyDeleteRachel, as noted above, argon is an infra-red active gas. But because it absorbs only in the near infra-red, it little affects IR emission from the surface of the Earth. What it does is cools the surface by preventing solar insolation from ever reaching the surface. Presumably, the greenhouse gases should be those that have some warming effect on the surface, at least under some conditions, and not include those gases that cool the surface, such as diatomic oxygen and argon.
ReplyDeleteHi
ReplyDeleteRef. fig 6
Where is the rest (30-25%) of 70-75% Transmitted Downgoing Solar Radiation and where is the rest (85-70%) of 15-30% Upgoing Thermal Radiation?
In Fig. 6, the solar radiation absorbed by the surface and atmosphere is the 70 - 75%, which is also what is transmitted through the atmosphere. The 25 - 30 % missing is reflected by clouds, aerosols, the top of the atmosphere, and the surface.
ReplyDeleteThe 15 - 30% upgoing thermal radiation is that from the surface that is emitted into space with absorption by the atmosphere. This is the thermal radiation said to be emitted through the atmospheric window in the wavelengths suitable for such purposes as infra-red guided weapons and sensors.
On the second part to be more clear, most of the thermal radiation emitted from the surface is absorbed by water vapor or other infra-red active gases in a very short distance as discussed in the article. Within a few tens of meters there is essentially no thermal radiation from the surface which has not been absorbed in the remainder of the surface thermal radiation spectrum, the missing 85 - 70%.
ReplyDeleteThank you for a most comprehensive and detailed paper, Charles.
ReplyDeleteIn addition I support your statement even more strongly with the conjecture that "These effects of added water vapor make it most unlikely that water vapor has a strong positive feedback effect upon increased carbon dioxide supposed warming. "
While there may well be a positive feedback loop in WV and surface layer temperature, it is limited to a very short distance above the surface and simply helps drive the conversion of the available energy into convectively transported WV latent heat to the necessary altitude where it participates in IR radiation to space.
Since WV provides some 5/6 of all the radiation source to space, any increase in WV production will increase the IR radiation to space. WV is thus by definition a negative feedback in the equation. As such the entire 'global warming' conjecture as postulated by the IPCC as dependent on WV positive feedback, collapses.
re. the Paul Dennis comment: " Feynman in his lectures (volume 1) in which he demonstrates that a static gas in a gravitational field is actually isothermal.."
ReplyDeleteI of course would never question Feynman. However due to the heating of the water vapor and lower atmosphere by energy from the surface, the strong convective forces ensure that this in not a 'static atmosphere' and therefore i don't believe that the isothermal comment applies.
Thanks for your comments Ronald. It is now firmly established that an increased surface temperature will cause an increase in water vapor in the atmosphere. At today's general water vapor concentrations, the net effect of more water vapor is a cooling effect at the surface. Consequently, if carbon dioxide increases were to cause a surface temperature increase, the water vapor feedback would be negative. However, it even appears that at present CO2 concentrations, the effect of further CO2 is also a small cooling effect.
ReplyDeleteVery interesting blog Charles, I have been looking for info on this for many years since I had a hunch the effect of GH gasses on incoming solar IR was being ignored.
ReplyDeleteOne clarification though, when you say 'This makes it appear that IR-active gas absorption is much more important an effect on the emission side than on the solar insolation side' you are talking about the x axis being logarithmic, hence compressing the chart on the right. Doesn't this make it look smaller and hence less important?
Also, the y axis on fig 2 is in w/m^2 um^-1 so the right hand side of the graph is flattened compared to if it was just w/m^2?
Don't you also think the cooling effect of GH gasses is only active during energy flow into the system, ie in the day up to Tmax, and that after this, when energy flow is out of the system, they have a warming effect?
@Rosco
ReplyDeleteRegarding the moon, since the minimum temperature is almost a flat line reached immediately on sun down, ie, the temperature graph shows a rapid drop off, then turns 90` left, don't you think the surface temperature is more a function of incident light than time? (Also Tmax appears to occur pretty much at peak insolation).
I checked my Feynman's Lectures Vol. 1. and he calculates a temperature gradient in a static atmosphere which is hardly isothermal. It is in fact a linear gradient because he assumes g is constant. Of course g decreases slowly with altitude.
ReplyDeleteIn Fig. 6., which shows the incoming solar energy and its absorptions and the outgoing Earth surface thermal radiation, the x-axis is logarithmic in the wavelength. Energy is inversely proportional to the wavelength. Thus, the energy of a photon would decrease rapidly as one moves right along the x-axis even if the x-axis were linear. Given that it is logarithmic, the energy of each photon emitted decreases even more rapidly.
ReplyDeleteThe question about Fig. 2. is not making sense to me. You may have meant Fig.4. You cannot plot power vs wavelength except for per a delta wavelength.
The cooling mechanism due to atmospheric absorption of incoming solar insolation works only during the day and hence tends to reduce the temperature differential between night and day. This is good. However, added CO2 above the altitudes of water vapor condensation is a cooling mechanism. CO2 cools all day long as a high specific heat molecule in rising convection currents. It heat capacity is 27% higher than that of nitrogen molecules, so it carries more heat upward. It also cools as it emits thermal radiation from a warmer layer of atmospheric gases to a cooler layer above it, since the speed of light is faster than the speed of convection currents. Each of these other cooling mechanisms works day or night. As one adds CO2 to the atmosphere, at the lowest concentrations it warms the atmosphere, but further additions cause less and less warming and then still more causes cooling and yet more still more cooling. This is because the degree to which CO2 prevents thermal radiation from leaving the atmosphere through the atmospheric window decreases logarithmically with additional CO2, while many of these cooling mechanisms saturate more slowly or not at all.
Yes, Rosco, the incident radiation on the moon is the most important factor.
ReplyDeleteHello Dr Anderson,
ReplyDeleteI am currently working through/ reading your work, but have skimmed it looking for similar keywords to my worked (Raman active spectra etc).
Maybe my two new papers will interest you? 'Quantum Mechanics and Raman Spectroscopy Refute Greenhouse Theory' and 'The Greenhouse Gases and Infrared Radiation Misconceived by Thermoelectric Transducers'.
I have shown N2 and O2 absorb and emit IR photons, and can prove it by experiment (Frank-Hertz experiment and the N2-CO2 laser where N2's QM predicted 2338cm-1 mode absorbs electrons or photons) and that the so-called GHGs are really only the thermoelectric gases - they also emit and absorb IR photons but are not special other than they are received by thermoelectric transducers - thermopiles originally (Tyndall, 1859).
I have uncovered a lot of other misconceptions relevant to radiation theory.
I hope to hear from you: fractalnomics at gmail.com
Blair
https://www.researchgate.net/publication/328927828_Quantum_Mechanics_and_Raman_Spectroscopy_Refute_Greenhouse_Theory https://www.researchgate.net/publication/329311153_The_Greenhouse_Gases_and_Infrared_Radiation_Misconceived_by_Thermoelectric_Transducers
or
https://www.academia.edu/37577750/Quantum_Mechanics_and_Raman_Spectroscopy_Refute_Greenhouse_Theory
or my
http://vixra.org/author/blair_d_macdonald
Hello Dr Anderson,
ReplyDeleteYou may be interested in my two complementary papers on quantum mechanics and the atmosphere? I think the future of atmospheric sciences is with Raman spectroscopy.
'Quantum Mechanics and Raman Spectroscopy Refute Greenhouse Theory' and 'The Greenhouse Gases and Infrared Radiation Misconceived by Thermoelectric Transducers'. I have shown N2 and O2 absorb and emit IR photons, can prove it by experiment (Frank-Hertz experiment and the N2-CO2 laser where N2's QM predicted 2338cm-1 mode absorbs electrons or photons) and that the so-called GHGs are really only the thermoelectric gases - they also emit and absorb IR photons but are not special other than they are received by thermoelectric transducers - thermopiles originally. I have uncovered a lot of other misconceptions relevant to radiation theory. https://www.researchgate.net/publication/328927828_Quantum_Mechanics_and_Raman_Spectroscopy_Refute_Greenhouse_Theory https://www.researchgate.net/publication/329311153_The_Greenhouse_Gases_and_Infrared_Radiation_Misconceived_by_Thermoelectric_Transducers
or
https://www.academia.edu/37577750/Quantum_Mechanics_and_Raman_Spectroscopy_Refute_Greenhouse_Theory
or my
http://vixra.org/author/blair_d_macdonald
Thank you for your significant contribution. I will read it in detail as I have been studying the related issues. Indeed, it is absurd to assume CO2 molecules completely emit all of the absorbed infrared radiation at 16 micrometer without transferring the energy into thermal energy.
ReplyDeleteIt is good work, but no more convincing than the IPCC. The nature of these systems is sufficient to expect that we should need to actually measure everything we can for multiple centuries. I don't believe we have had decent data for the subject for more than 50 years. Without a bunch of speculatory math, but with reference to the interpretations of the climates that produce the geologies through aeons, it seems apparent that temperatures like today have existed with 3,000ppm CO2 though most of Earth history is warmer than today as well as the average amount of CO2.
ReplyDeleteWe have got a lot of problems, but CO2 isn't high on that list in it's self. Really, I think it is a "sales tag" for a constellation of issues being sold.